Can Propane Form Isomers
Can Propane Form Isomers - However, c 4 h 10, has more than possible structure. There are also endless other possible ways that this molecule could twist itself. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that. Web the first two isomers shown of are propanols, that is, alcohols derived from propane. Propane is a hydrocarbon with chemical formula c 3 h 8 and is represented as follows: One way to think about this is as follows: Web propene (see figure below) has no geometric isomers because one of the carbon atoms (the one on the far left) involved in the double bond has two single hydrogens bonded to it. You can demonstrate this to yourself by drawing all possible structures for propane (1), butanes (2), pentanes (3), and hexanes (5). Molecules that have the same molecular formula but different molecular geometries are called isomers. Web they are not isomers.
Option b is the correct answer. There are also endless other possible ways that this molecule could twist itself. Web they are not isomers. Isomerism is defined as the phenomenon in which more than one compounds have the same chemical formula. Molecules that have the same molecular formula but different molecular geometries are called isomers. Web so the answer to the question that can you make isomers of propane, is false. Web the first two isomers shown of are propanols, that is, alcohols derived from propane. Each carbon you add can attach to any of the carbons already present in any isomer of the molecule. But the main fact is, the chemical structures of the compounds are different. You can demonstrate this to yourself by drawing all possible structures for propane (1), butanes (2), pentanes (3), and hexanes (5).
Web they are not isomers. Propane is a hydrocarbon with chemical formula c 3 h 8 and is represented as follows: Molecules that have the same molecular formula but different molecular geometries are called isomers. Web so the answer to the question that can you make isomers of propane, is false. Each carbon you add can attach to any of the carbons already present in any isomer of the molecule. Web the molecular geometries of hydrocarbons are directly related to the physical and chemical properties of these molecules. Option b is the correct answer. There are two major classes of isomers: Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. Web propene (see figure below) has no geometric isomers because one of the carbon atoms (the one on the far left) involved in the double bond has two single hydrogens bonded to it.
Organic Molecules and Isomers Biology 201 The Chemistry of Life
However, c 4 h 10, has more than possible structure. Isomerism is defined as the phenomenon in which more than one compounds have the same chemical formula. There are also endless other possible ways that this molecule could twist itself. Option b is the correct answer. Web the first two isomers shown of are propanols, that is, alcohols derived from.
Conformational Isomers of Propane Master Organic Chemistry
Web propene (see figure below) has no geometric isomers because one of the carbon atoms (the one on the far left) involved in the double bond has two single hydrogens bonded to it. Web there are no isomers of propane because its structure shows that it lacks enough carbon atoms to exist in the form of a branching isomer. Molecules.
Enantiomers Chemistry Steps
Isomerism is defined as the phenomenon in which more than one compounds have the same chemical formula. There are two major classes of isomers: Propane is a hydrocarbon with chemical formula c 3 h 8 and is represented as follows: However, c 4 h 10, has more than possible structure. Web they are not isomers.
Longhorn Propane Residential Propane Request Form
There are two major classes of isomers: If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that. Web there are no isomers of propane because its structure shows that it lacks enough carbon atoms to exist in the.
Conformational Isomers of Propane YouTube
Web so the answer to the question that can you make isomers of propane, is false. Molecules that have the same molecular formula but different molecular geometries are called isomers. But the main fact is, the chemical structures of the compounds are different. If you had a model of a molecule in front of you, you would have to take.
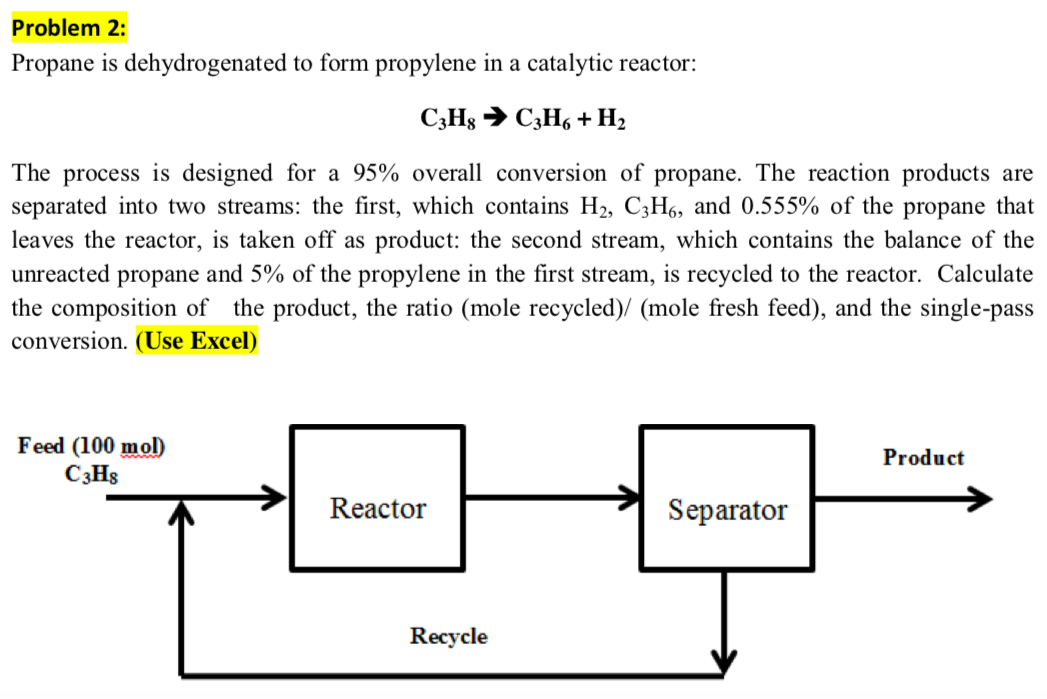
Solved Problem 2 Propane is dehydrogenated to form
Propane is a hydrocarbon with chemical formula c 3 h 8 and is represented as follows: Web so the answer to the question that can you make isomers of propane, is false. Molecules that have the same molecular formula but different molecular geometries are called isomers. Each carbon you add can attach to any of the carbons already present in.
7 Isomers Examples in Daily Life StudiousGuy
Both have a chain of three carbon atoms connected by single bonds, with the remaining carbon valences being filled by seven hydrogen atoms and by a hydroxyl group comprising the oxygen atom bound to a hydrogen atom. Each carbon you add can attach to any of the carbons already present in any isomer of the molecule. But the main fact.
Conformational Isomers of Propane LaptrinhX / News
Option b is the correct answer. There are also endless other possible ways that this molecule could twist itself. Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. However, c 4 h 10, has more than possible structure. Propane is a hydrocarbon with chemical formula c 3 h 8 and is represented.
Conformational Isomers of Propane Master Organic Chemistry
You can demonstrate this to yourself by drawing all possible structures for propane (1), butanes (2), pentanes (3), and hexanes (5). However, c 4 h 10, has more than possible structure. Web there are no isomers of propane because its structure shows that it lacks enough carbon atoms to exist in the form of a branching isomer. From the structure,.
Propane Gas Check Forms 20202022 Fill and Sign Printable Template
Each carbon you add can attach to any of the carbons already present in any isomer of the molecule. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that. Web propene (see figure below) has no geometric isomers.
Molecules That Have The Same Molecular Formula But Different Molecular Geometries Are Called Isomers.
Web the molecular geometries of hydrocarbons are directly related to the physical and chemical properties of these molecules. Physical and chemical properties of geometric isomers are generally different. Web generally the number of isomers increases. Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas.
Web The First Two Isomers Shown Of Are Propanols, That Is, Alcohols Derived From Propane.
But the main fact is, the chemical structures of the compounds are different. However, c 4 h 10, has more than possible structure. There are two major classes of isomers: Web they are not isomers.
One Way To Think About This Is As Follows:
Each carbon you add can attach to any of the carbons already present in any isomer of the molecule. Option b is the correct answer. You can demonstrate this to yourself by drawing all possible structures for propane (1), butanes (2), pentanes (3), and hexanes (5). There are also endless other possible ways that this molecule could twist itself.
Web Propene (See Figure Below) Has No Geometric Isomers Because One Of The Carbon Atoms (The One On The Far Left) Involved In The Double Bond Has Two Single Hydrogens Bonded To It.
Propane is a hydrocarbon with chemical formula c 3 h 8 and is represented as follows: From the structure, we can say that each carbon molecule must have four bonds. Both have a chain of three carbon atoms connected by single bonds, with the remaining carbon valences being filled by seven hydrogen atoms and by a hydroxyl group comprising the oxygen atom bound to a hydrogen atom. Web so the answer to the question that can you make isomers of propane, is false.