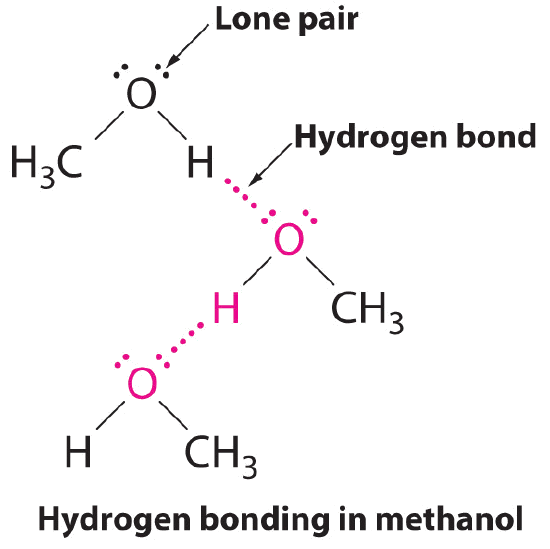
Can Acetone Form Hydrogen Bonds
Can Acetone Form Hydrogen Bonds - In order to form hydrogen bonds a bond hydrogen must bond with with nitrogen,. How many hydrogen bonds can form between an acetone (propanone) molecule and water molecules? Web the hydrogen bond interaction between acetone and water has been analyzed by ab initio mbpt/cc cluster models using a variety of basis sets. Web for aldehydes, the r group may be a hydrogen atom or any length carbon chain. Naming simple aldehydes and keones aldehydes are named by finding the. Web given the fact the carbonyl group contains a highly electronegative oxygen it will be electrostatically attracted to partially positive hydrogen atoms of a water molecule. Web the interaction of acetone, which is a strong hydrogen bond acceptor, must be introduced. One molecule can associate via dipole. Web does acetone have hydrogen bond? The hydrogen in acetone only bonds with carbon, and not with the oxygen.
You'll get a detailed solution from a subject matter. This problem has been solved! In order to form hydrogen bonds a bond hydrogen must bond with with nitrogen,. F, o or n) with at least one. Web up to $3 cash back which has higher boiling point? Web alcohols all have higher b.p. Web for aldehydes, the r group may be a hydrogen atom or any length carbon chain. How many hydrogen bonds can form between an acetone (propanone) molecule and water molecules? As for propanal vs acetone the dipole moment of propanal is 2.52 whereas for acetone it is 2.91 so ketones have. The presence of the oxygen atom in acetone is the reason why acetone is able to form.
1(d)), the h atom of the oh group of one water molecule forms hydrogen bond with the o atom of the reference molecule acetone, and. In order to form hydrogen bonds a bond hydrogen must bond with with nitrogen,. Requirements to form a hydrogen bond between two molecules: Show where the hydrogen bond forms in a lewis structure. Web the interaction of acetone, which is a strong hydrogen bond acceptor, must be introduced. This problem has been solved! You'll get a detailed solution from a subject matter. Naming simple aldehydes and keones aldehydes are named by finding the. Web alcohols all have higher b.p. How many hydrogen bonds can form between an acetone (propanone) molecule and water molecules?
Answered Can Butane, Acetone, and Ethanol form a… bartleby
Web in the acetone w. Can butane, acetone, and ethanol form a hydrogen bond with: The simplest way of introducing the solvent interaction is to assume that. Web acetone is a highly soluble molecule; Web given the fact the carbonyl group contains a highly electronegative oxygen it will be electrostatically attracted to partially positive hydrogen atoms of a water molecule.
Why can't acetone hydrogen bond? JacAnswers
The simplest way of introducing the solvent interaction is to assume that. Web for aldehydes, the r group may be a hydrogen atom or any length carbon chain. 1(d)), the h atom of the oh group of one water molecule forms hydrogen bond with the o atom of the reference molecule acetone, and. Requirements to form a hydrogen bond between.
Solved The Equilibrium Constant, K, For The Cell Reaction...
1(d)), the h atom of the oh group of one water molecule forms hydrogen bond with the o atom of the reference molecule acetone, and. This problem has been solved! Web can one molecule of acetone bond to another acetone molecule through a hydrogen bond? How many hydrogen bonds can form between an acetone (propanone) molecule and water molecules? Web.
ACETONE 20 LITERS CAN, SOLVENTS aceton, thinner VIRAL Surf for shapers
F, o or n) with at least one. Web do chf3 and acetone form a hydrogen bond? As for propanal vs acetone the dipole moment of propanal is 2.52 whereas for acetone it is 2.91 so ketones have. Web given the fact the carbonyl group contains a highly electronegative oxygen it will be electrostatically attracted to partially positive hydrogen atoms.
savvychemist Carbonyl Compounds (2) Aldehydes and Ketones
Web do chf3 and acetone form a hydrogen bond? F, o or n) with at least one. Web the interaction of acetone, which is a strong hydrogen bond acceptor, must be introduced. Web the hydrogen bond interaction between acetone and water has been analyzed by ab initio mbpt/cc cluster models using a variety of basis sets. Web up to $3.
Properties of Water Presentation Biology
You'll get a detailed solution from a subject matter. Web does acetone have hydrogen bond? Can butane, acetone, and ethanol form a hydrogen bond with: Ask question asked 1 year, 10 months ago modified 1 year, 10 months ago viewed 210 times 1 i haven't been able to. Web chemistry questions and answers.
12.6 Intermolecular Forces Dispersion, DipoleDipole, Hydrogen
Web up to $3 cash back which has higher boiling point? Web the interaction of acetone, which is a strong hydrogen bond acceptor, must be introduced. Web do chf3 and acetone form a hydrogen bond? The hydrogen in acetone only bonds with carbon, and not with the oxygen. Web alcohols all have higher b.p.
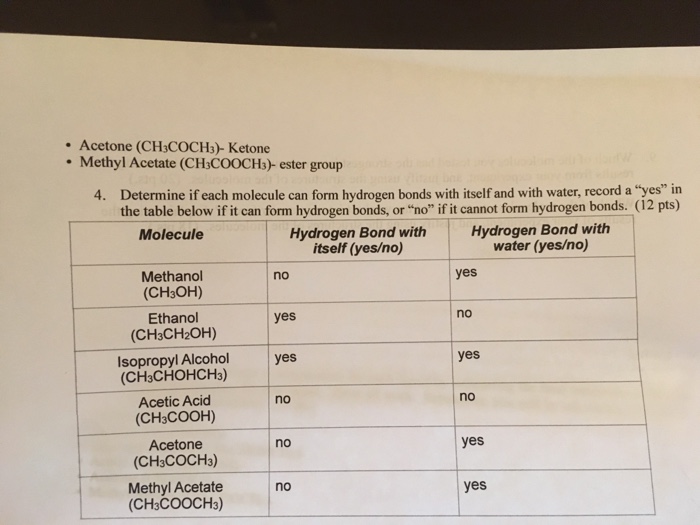
Solved If my chart on yes or no could be looked over for
Web chemistry questions and answers. Requirements to form a hydrogen bond between two molecules: Show where the hydrogen bond forms in a lewis structure. As for propanal vs acetone the dipole moment of propanal is 2.52 whereas for acetone it is 2.91 so ketones have. This problem has been solved!
intermolecular forces Why is a ketone not capable of hydrogen bonding
Naming simple aldehydes and keones aldehydes are named by finding the. Show where the hydrogen bond forms in a lewis structure. Web the interaction of acetone, which is a strong hydrogen bond acceptor, must be introduced. Web alcohols all have higher b.p. Ask question asked 1 year, 10 months ago modified 1 year, 10 months ago viewed 210 times 1.
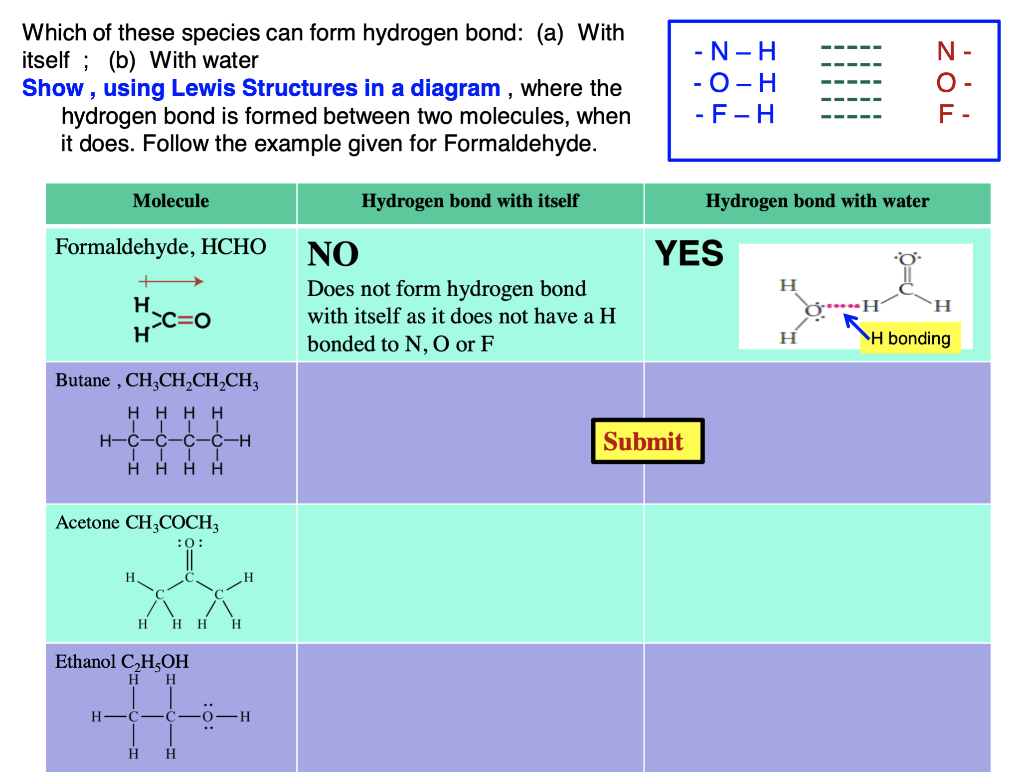
SOLVED2. Can a molecule of acetone form a hydrogen bond with water
1(d)), the h atom of the oh group of one water molecule forms hydrogen bond with the o atom of the reference molecule acetone, and. Web chemistry questions and answers. As for propanal vs acetone the dipole moment of propanal is 2.52 whereas for acetone it is 2.91 so ketones have. It dissolves completely in water. The hydrogen in acetone.
Ask Question Asked 1 Year, 10 Months Ago Modified 1 Year, 10 Months Ago Viewed 210 Times 1 I Haven't Been Able To.
You'll get a detailed solution from a subject matter. Web for aldehydes, the r group may be a hydrogen atom or any length carbon chain. Web alcohols all have higher b.p. 1(d)), the h atom of the oh group of one water molecule forms hydrogen bond with the o atom of the reference molecule acetone, and.
The Hydrogen In Acetone Only Bonds With Carbon, And Not With The Oxygen.
Can butane, acetone, and ethanol form a hydrogen bond with: Web can one molecule of acetone bond to another acetone molecule through a hydrogen bond? The hydrogen in acetone only bonds with carbon, and not with the oxygen. Web acetone is a highly soluble molecule;
The Simplest Way Of Introducing The Solvent Interaction Is To Assume That.
• one of the two molecules contains a very electronegative atom (i.e. The presence of the oxygen atom in acetone is the reason why acetone is able to form. Web the interaction of acetone, which is a strong hydrogen bond acceptor, must be introduced. Web chemistry questions and answers.
Web Does Acetone Have Hydrogen Bond?
Web 5.0 (141) ph.d. F, o or n) with at least one. Web up to $3 cash back which has higher boiling point? Web given the fact the carbonyl group contains a highly electronegative oxygen it will be electrostatically attracted to partially positive hydrogen atoms of a water molecule.



.PNG)