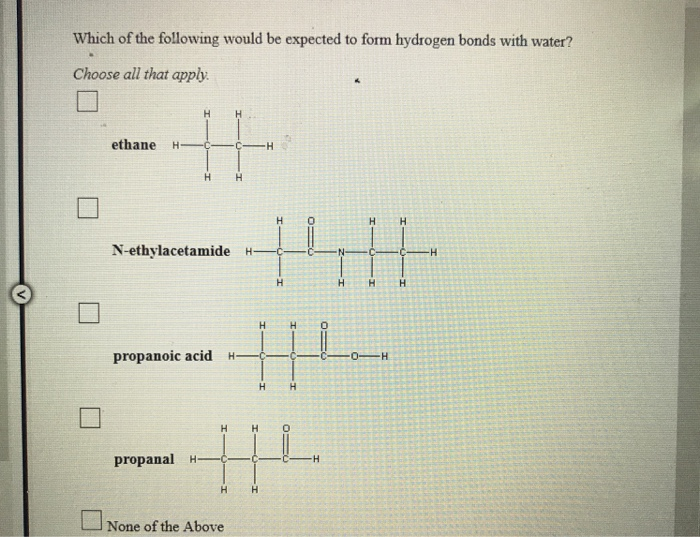
Will Ethane Form A Hydrogen Bond With Water
Will Ethane Form A Hydrogen Bond With Water - Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because ethane is nonpolar. Web same thing with this one, once it vaporizes, it's out in gaseous state, it's much further from any other water molecules, it's not going to be able to form those hydrogen bonds with. No ethane (c2h6) is a hydrocarbon and hydrocarbons can't form hydrogen bonds. But fitting the ether molecules into the water solvent. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. In h 2 o, only two of the six. Is an ethane molecule a. C2h4(g) + 3o2(g) → 2co2(g) + 2h2o (l). Web the hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. It has the chemical formula ch.
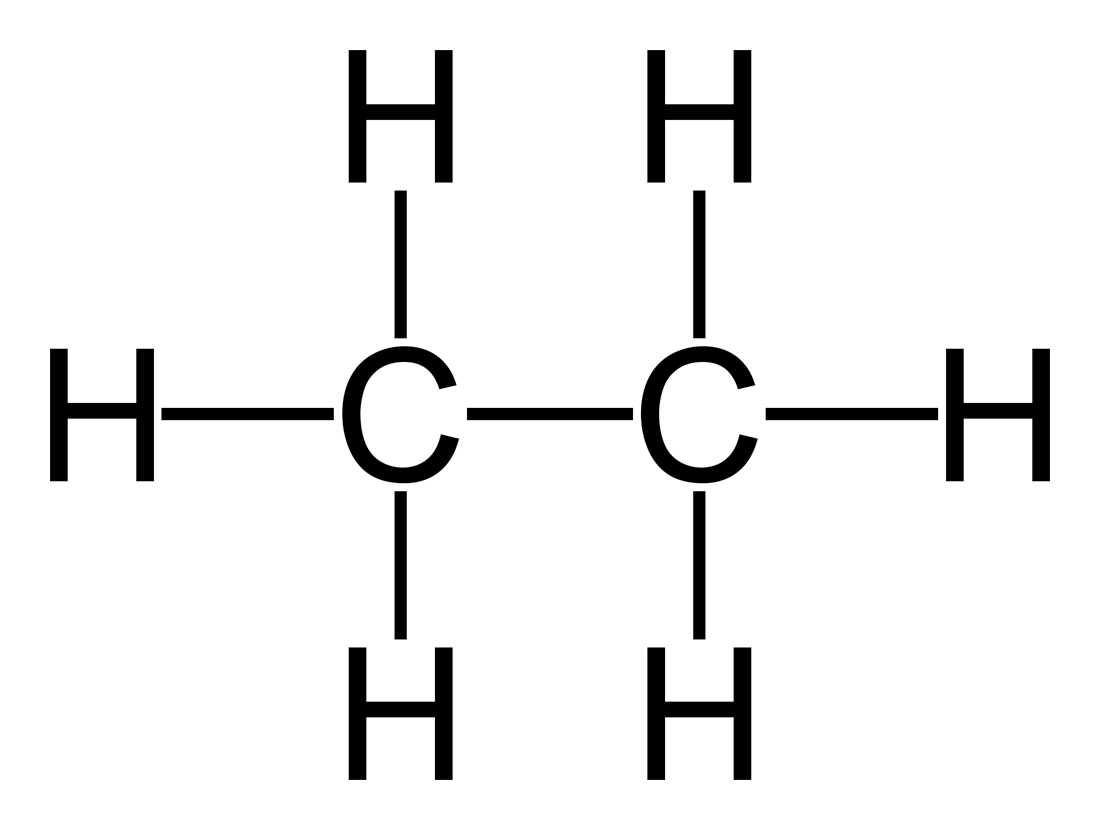
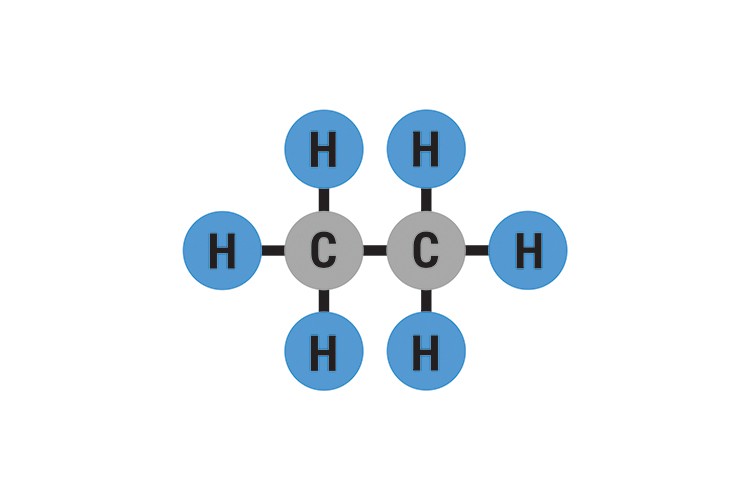
Web the hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. Web the chemical formula of ethane? • broad range of geometric hydrogen bond definitions is. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because ethane is nonpolar. The two carbon atoms bond by merging their remaining sp 3. The methane molecule provides an example: C2h6(g) + 3½o2(g) → 2co2(g) + 3h2o (l) ethene + oxygen → carbon dioxide + water. Web ethoxyethane, better known as diethyl ether or even just ether, can form hydrogen bonds with water. Web same thing with this one, once it vaporizes, it's out in gaseous state, it's much further from any other water molecules, it's not going to be able to form those hydrogen bonds with.
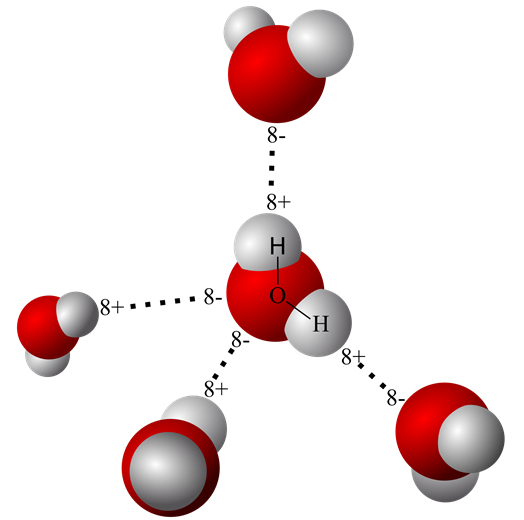
In h 2 o, only two of the six. • broad range of geometric hydrogen bond definitions is. The two carbon atoms bond by merging their remaining sp 3. We investigated factors that could affect the hydrogen. Web the hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Oxygen is highly electronegative, which creates a partial negative charge on one end of. What is the intramolecular bond that holds the hydrogen and carbon atoms within an ethane molecule? Web ethoxyethane, better known as diethyl ether or even just ether, can form hydrogen bonds with water. Web the chemical formula of ethane?
Solved Which of the following would be expected to form
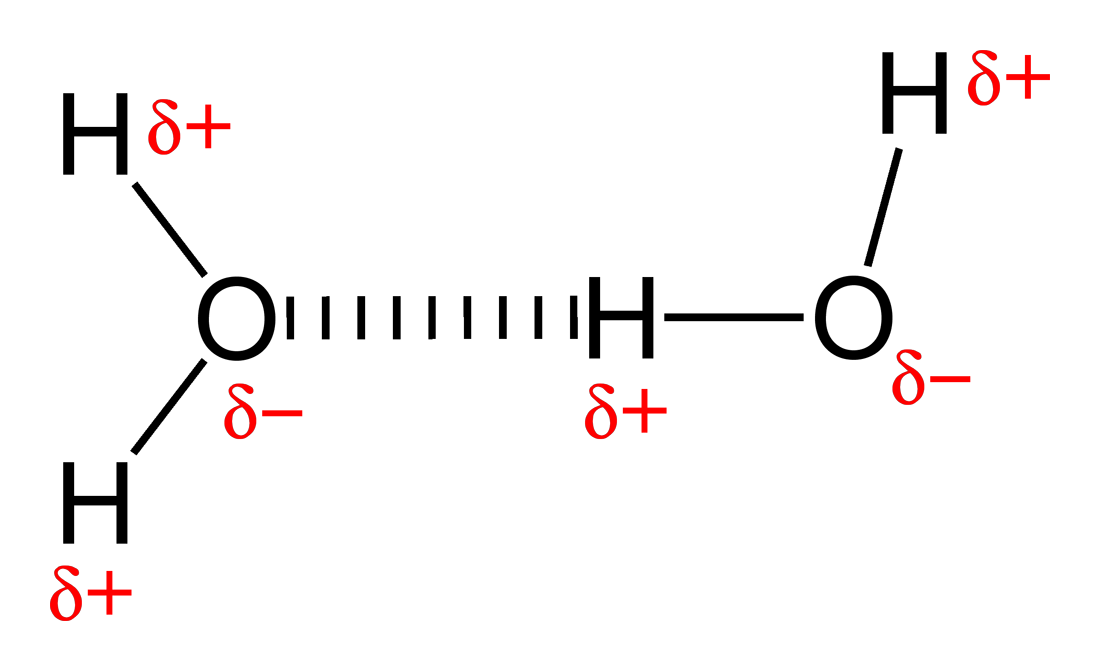
Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Is an ethane molecule a. C2h6(g) + 3½o2(g) → 2co2(g) + 3h2o (l) ethene + oxygen → carbon dioxide + water. It has the chemical formula ch. • broad range of geometric hydrogen bond definitions is.
8.1 Intermolecular Interactions The Basics of General, Organic, and
The methane molecule provides an example: We investigated factors that could affect the hydrogen. • broad range of geometric hydrogen bond definitions is. But fitting the ether molecules into the water solvent. C2h6(g) + 3½o2(g) → 2co2(g) + 3h2o (l) ethene + oxygen → carbon dioxide + water.
What is a hydrogen bond? [With free chemistry study guide]
It has the chemical formula ch. In h 2 o, only two of the six. Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. C2h4(g) + 3o2(g) → 2co2(g) + 2h2o (l). Web the chemical formula of ethane?
Solved Below are three molecules, hexan3one,
• broad range of geometric hydrogen bond definitions is. Web ethane + oxygen → carbon dioxide + water. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. But fitting the ether molecules into the water solvent. What is the intramolecular bond that holds the hydrogen and carbon atoms within an.
How many covalent bonds are there in a molecule of class 12 chemistry CBSE
Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because ethane is nonpolar. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Web water is made up of two.
The molecular structure of Ethane and formula structure
C2h6(g) + 3½o2(g) → 2co2(g) + 3h2o (l) ethene + oxygen → carbon dioxide + water. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web same thing with this one, once it vaporizes, it's out in gaseous.
Article 167 The Geometry of Molecules Part 2 Visual Library
Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. It has the chemical formula ch. Web the chemical formula of ethane? No ethane (c2h6) is a hydrocarbon and hydrocarbons can't form hydrogen bonds. In h 2 o, only two of the six.
Pin on Hydrogen
Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Web ethane + oxygen → carbon dioxide + water. C2h6(g) + 3½o2(g) → 2co2(g) + 3h2o (l) ethene + oxygen → carbon dioxide + water. Oxygen is highly electronegative, which creates a partial negative charge on.
The Curious Wavefunction A bond by any other name... How the simple
Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web will ethane form a hydrogen bond with water? Is an ethane molecule a. The two carbon atoms bond by merging their remaining sp 3. Oxygen is highly electronegative, which creates a partial negative charge on one end of.
hydrophilic vs. hydrophobic
Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because ethane is nonpolar. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web the chemical formula of ethane? We investigated factors that could affect the hydrogen. Web will ethane form a hydrogen bond with water?
Oxygen Is Highly Electronegative, Which Creates A Partial Negative Charge On One End Of.
C2h6(g) + 3½o2(g) → 2co2(g) + 3h2o (l) ethene + oxygen → carbon dioxide + water. The two carbon atoms bond by merging their remaining sp 3. Web will ethane form a hydrogen bond with water? Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k.
Web Ethane + Oxygen → Carbon Dioxide + Water.
Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Web ethoxyethane, better known as diethyl ether or even just ether, can form hydrogen bonds with water. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. • broad range of geometric hydrogen bond definitions is.
But Fitting The Ether Molecules Into The Water Solvent.
Is an ethane molecule a. No ethane (c2h6) is a hydrocarbon and hydrocarbons can't form hydrogen bonds. In h 2 o, only two of the six. We investigated factors that could affect the hydrogen.
Web The Hydrogens Bond With The Two Carbons To Produce Molecular Orbitals Just As They Did With Methane.
It has the chemical formula ch. C2h4(g) + 3o2(g) → 2co2(g) + 2h2o (l). The methane molecule provides an example: Web other common substances which are freely soluble in water because they can hydrogen bond with water molecules include ethanol (alcohol) and sucrose.

![What is a hydrogen bond? [With free chemistry study guide]](http://www.aceorganicchem.com/blog/wp-content/uploads/2018/04/4-22-2018-6-20-10-PM.jpg)