What Types Of Ions Do Metals Form
What Types Of Ions Do Metals Form - Most atoms do not have eight electrons. Web you’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Positively charged ions are called cations; Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. Web the chemical differences between metals and nonmetals that interest us the most: Web anonymous libretexts learning objectives define the two types of ions. Metals tend to form cations and nonmetals tend to form anions. Web metal atoms lose electrons from their outer shell when they form ions: Use lewis diagrams to illustrate ion formation. As you also have heard them as transition metals.
Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. Web the chemical differences between metals and nonmetals that interest us the most: Positively charged ions are called cations; Carbon (c) and silicon (si) are nonmetals that rarely form. Most atoms do not have eight electrons. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web nonmetals form negatively charged ions, or anions. The ions are positive, because they have more protons than electrons the ions formed have full outer. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. Web you’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges.
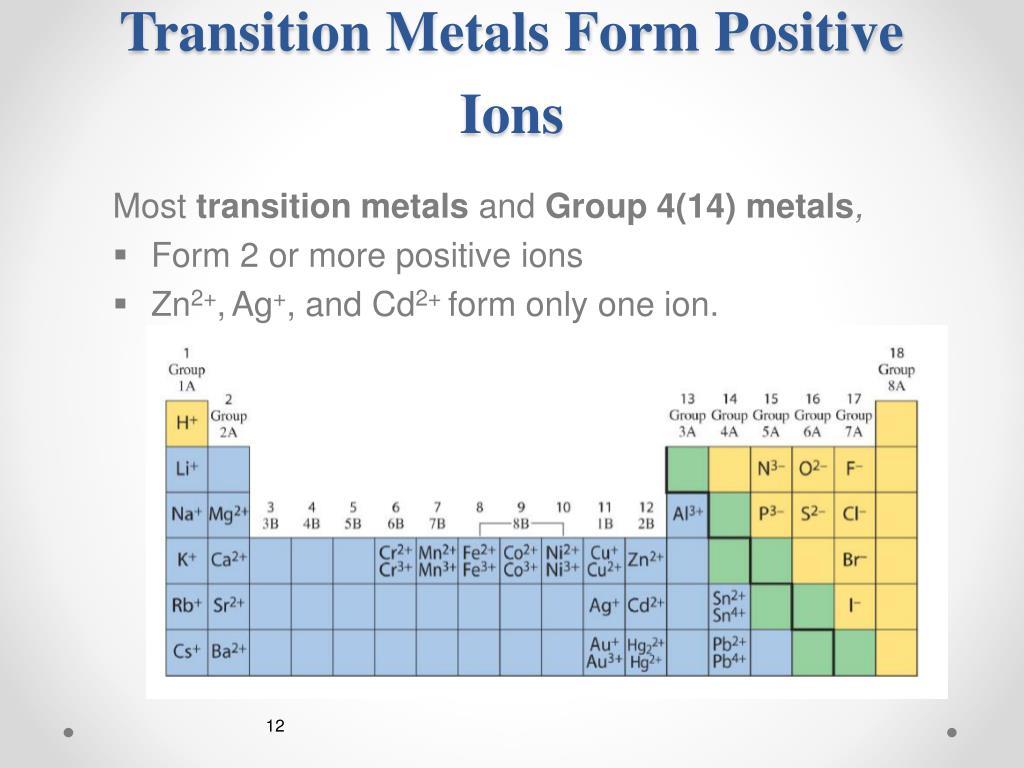
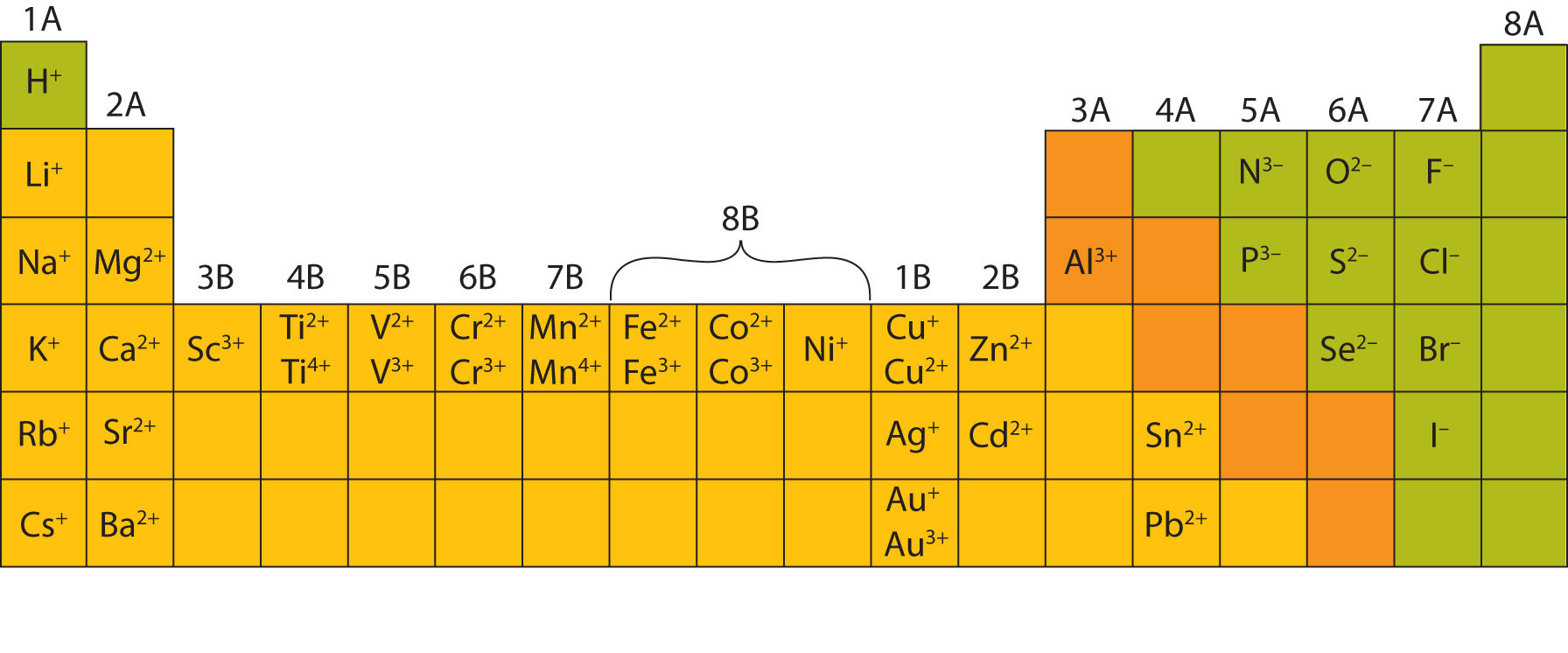
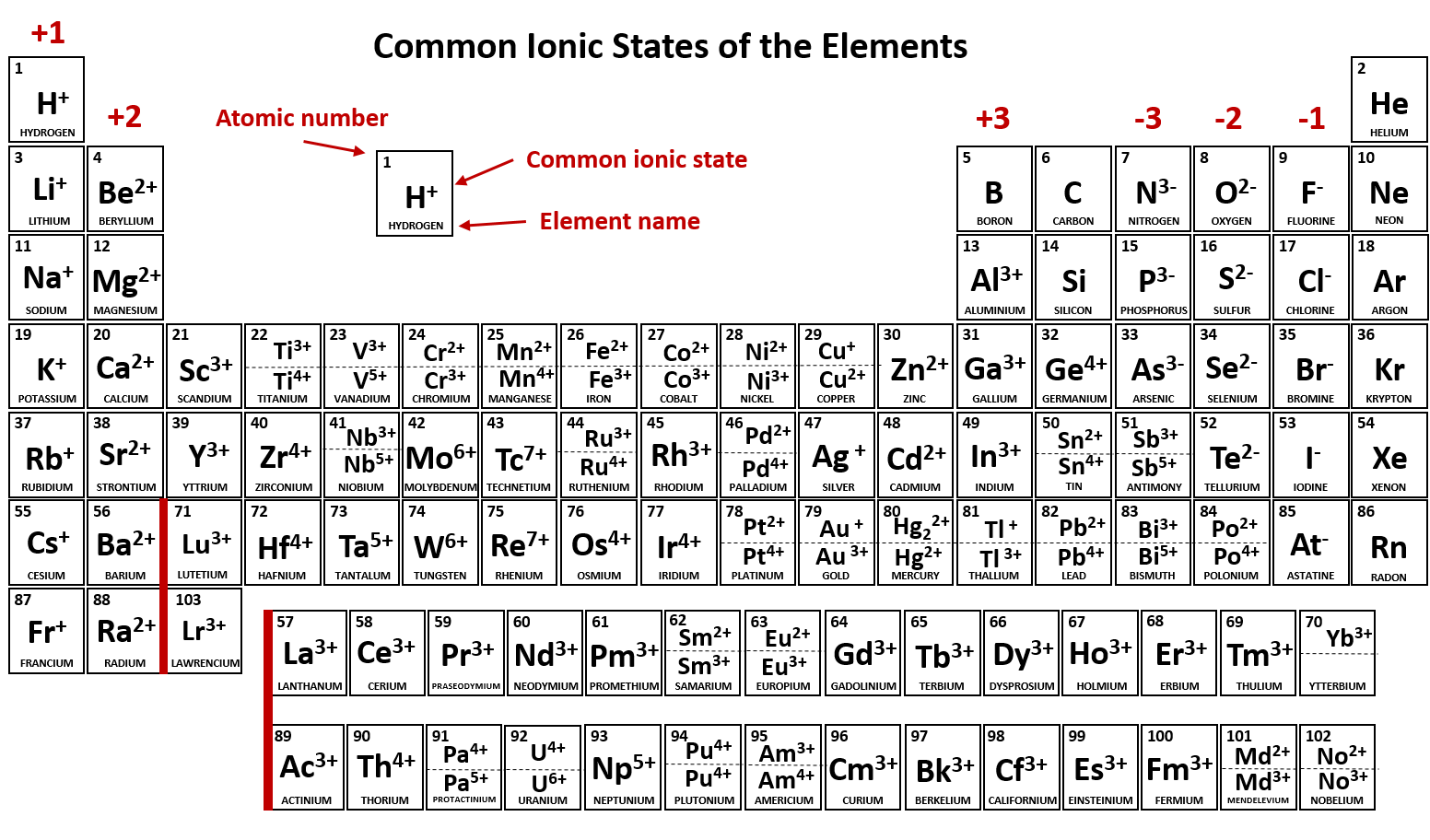
An iron(ii) ion has a 2+ charge, and an iron(iii) ion has a 3+ charge. Metals tend to form cations and nonmetals tend to form anions. The ions are positive, because they have more protons than electrons the ions formed have full outer. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web answered • expert verified. The following periodic table shows some of the common ions formed by. What type of ions do metals naturally form? Web nonmetals form negatively charged ions, or anions. Carbon (c) and silicon (si) are nonmetals that rarely form. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Most atoms do not have eight electrons. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. Web metal atoms lose electrons from their outer shell when they form ions: Metals tend to form cations and nonmetals tend to form anions. As you also have heard them.
PPT Naming Ionic Compounds PowerPoint Presentation, free download
The following periodic table shows some of the common ions formed by. The scientific name for positively charged ions is cations. What type of ions do metals naturally form? They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons,. Web group iv a metals form cations with a +4.
Chem matters ch6_ionic_bond
Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. Carbon (c) and silicon (si) are nonmetals that rarely form. Web the type of ions that metals form are called positively charged ions. The ions are positive, because they have more protons than electrons the ions formed.
Chem matters ch6_ionic_bond
Use lewis diagrams to illustrate ion formation. Web chemistry matter elements 1 answer umair.a jul 3, 2016 they form cations (positively charged ion). Carbon (c) and silicon (si) are nonmetals that rarely form. Web nonmetals form negatively charged ions, or anions. An iron(ii) ion has a 2+ charge, and an iron(iii) ion has a 3+ charge.
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free
Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. The ions are positive, because they have more protons than electrons the ions formed have full outer. They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons,. An iron(ii) ion has a.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
What type of ions do metals naturally form? Web group iv a metals form cations with a +4 charge, whereas tin (sn) and lead (pb) can form cations with a +2 charge. Positively charged ions are called cations; Metals tend to form cations and nonmetals tend to form anions. Web nonmetals form negatively charged ions, or anions.
PPT 1 Name the ions formed by these elements and classify them as
Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web chemistry matter elements 1 answer umair.a jul 3, 2016 they form cations (positively charged ion). Web the chemical differences between metals and nonmetals that interest us the most: Web group iv a metals form cations with a +4 charge, whereas tin (sn).
Ionic Bonding and Simple Ionic Compounds
Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. Use lewis diagrams to illustrate ion formation. Metals tend to form cations and nonmetals tend to form anions. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. An iron(ii) ion has a 2+ charge, and an iron(iii) ion.
Ionic Bond Definition, Types, Properties & Examples
The following periodic table shows some of the common ions formed by. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. Web answered • expert verified. Negative ions, by gaining electrons to fill the valence shell negative ions, by losing. The ions are positive, because they.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
The ions are positive, because they have more protons than electrons the ions formed have full outer. Web metal atoms lose electrons from their outer shell when they form ions: What type of ions do metals naturally form? Web the chemical differences between metals and nonmetals that interest us the most: Use lewis diagrams to illustrate ion formation.
As You Also Have Heard Them As Transition Metals.
An iron(ii) ion has a 2+ charge, and an iron(iii) ion has a 3+ charge. Carbon (c) and silicon (si) are nonmetals that rarely form. The following periodic table shows some of the common ions formed by. Web metal atoms lose electrons from their outer shell when they form ions:
Web Chemistry Matter Elements 1 Answer Umair.a Jul 3, 2016 They Form Cations (Positively Charged Ion).
Most atoms do not have eight electrons. Web the chemical differences between metals and nonmetals that interest us the most: The scientific name for positively charged ions is cations. Web answered • expert verified.
They Do This Because They Need To Gain One To Three Electrons In Order To Achieve An Octet Of Valence Electrons,.
Web nonmetals form negatively charged ions, or anions. Positively charged ions are called cations; The ions are positive, because they have more protons than electrons the ions formed have full outer. Metals tend to form cations and nonmetals tend to form anions.
Web Anonymous Libretexts Learning Objectives Define The Two Types Of Ions.
Web you’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Web group iv a metals form cations with a +4 charge, whereas tin (sn) and lead (pb) can form cations with a +2 charge. Web the type of ions that metals form are called positively charged ions. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant.