What Types Of Elements Form Ionic Compounds
What Types Of Elements Form Ionic Compounds - Web ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist. Ionic compounds form by two ways: Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. The two most basic types of. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web compounds formed from positive and negative ions are called ionic compounds. Ions form when atoms lose or gain electrons to obtain a full outer shell:. Web compounds that contain ions are called ionic compounds.
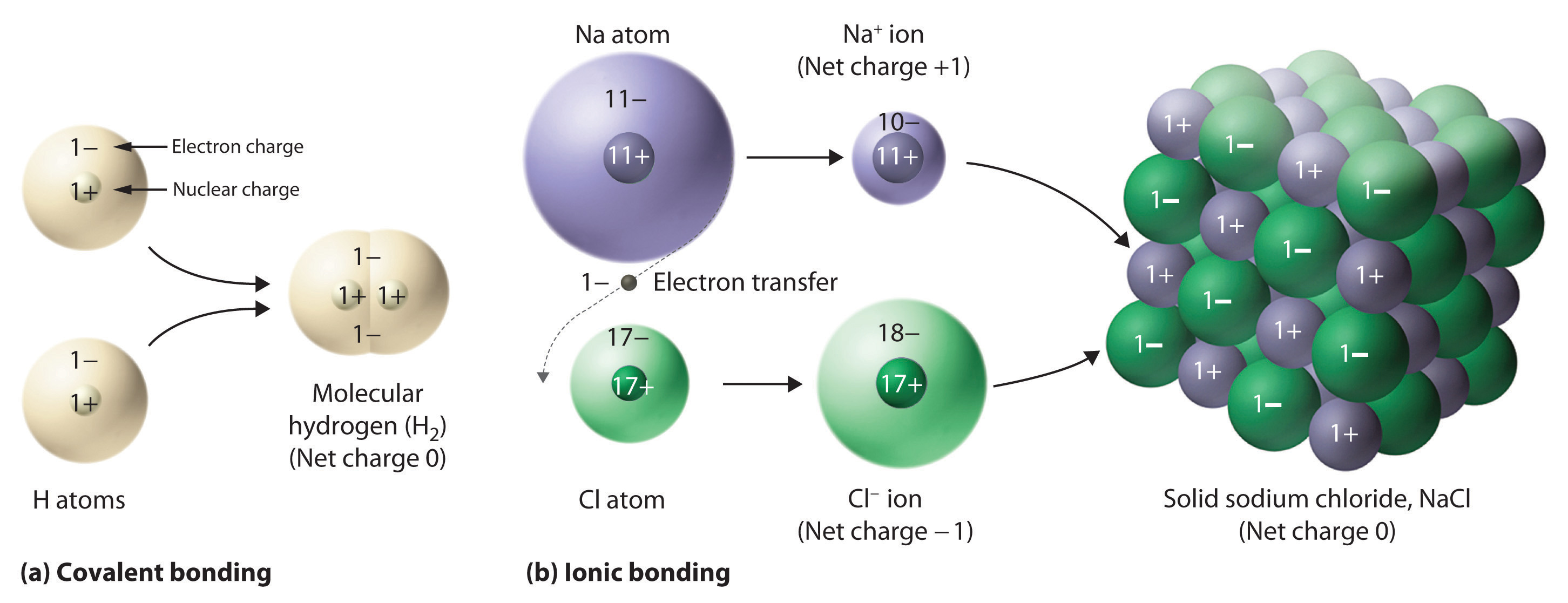
When they do, they become monatomicions. Web compounds that contain ions are called ionic compounds. Ionic compounds form by two ways: Ionic compounds form as electrons move from metal atoms to. Individual atoms can gain or lose electrons. Web ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist. Ions form when atoms lose or gain electrons to obtain a full outer shell:. Compounds between metal and nonmetal elements are usually ionic. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together.
Ionic compounds form as electrons move from metal atoms to. When they do, they become monatomicions. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist. Compounds between metal and nonmetal elements are usually ionic. Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Web compounds that contain ions are called ionic compounds. Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. Compounds that do not contain ions, but instead consist of.
Ionic Bond Definition, Types, Properties & Examples
To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web ionic compounds generally form from metals and nonmetals. Web ionic compounds consist of positively and negatively charged ions held together.
Periodic Table Ions List Periodic Table Timeline
Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web compounds that contain ions are called ionic compounds. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Individual atoms can gain or lose electrons. Compounds that.
Naming Simple Ionic Compounds Pathways to Chemistry
Then, identify the anion and write down its symbol and. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Ionic compounds form as electrons move from metal atoms to. Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or.
Examples of Ionic Bonds and Compounds
The two most basic types of. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Ions form when atoms lose or gain electrons to obtain a full outer shell:. For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Web ionic compounds are.
Ionic Bond Definition, Types, Properties & Examples
Compounds between metal and nonmetal elements are usually ionic. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Web ionic compounds are pure substances consisting of chemically bonded.
2.7 Ions and Ionic Compounds Chemistry LibreTexts
Individual atoms can gain or lose electrons. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. Web ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of. Compounds between metal and nonmetal elements are usually ionic.
Ionic Bond Definition, Types, Properties & Examples
Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web ionic compounds consist of positively.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
The two most basic types of. Web compounds formed from positive and negative ions are called ionic compounds. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Ionic compounds form by two ways: Web ionic compounds are pure substances consisting of chemically bonded ions.
Ionic bonding Wikipedia
Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web ionic compounds are pure substances consisting of chemically bonded ions. Web compounds that contain ions are called ionic compounds. Web ionic compounds generally form from metals and nonmetals. Ions form when atoms lose or gain.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Then, identify the anion and.
Ions Form When Atoms Lose Or Gain Electrons To Obtain A Full Outer Shell:.
Then, identify the anion and write down its symbol and. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,.
Ionic Compounds Form As Electrons Move From Metal Atoms To.
Compounds that do not contain ions, but instead consist of. Web compounds that contain ions are called ionic compounds. The two most basic types of. Ionic compounds generally form from metals and nonmetals.
Web When Atoms Of Nonmetal Elements Form Ions, They Generally Gain Enough Electrons To Give Them The Same Number Of Electrons As An Atom Of The Next Noble Gas In The Periodic.
When they do, they become monatomicions. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Compounds between metal and nonmetal elements are usually ionic.
Web Compounds Formed From Positive And Negative Ions Are Called Ionic Compounds.
Ionic compounds form by two ways: Web ionic compounds generally form from metals and nonmetals. Individual atoms can gain or lose electrons. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table.



/ionic-bond-58fd4ea73df78ca1590682ad.jpg)




.PNG)