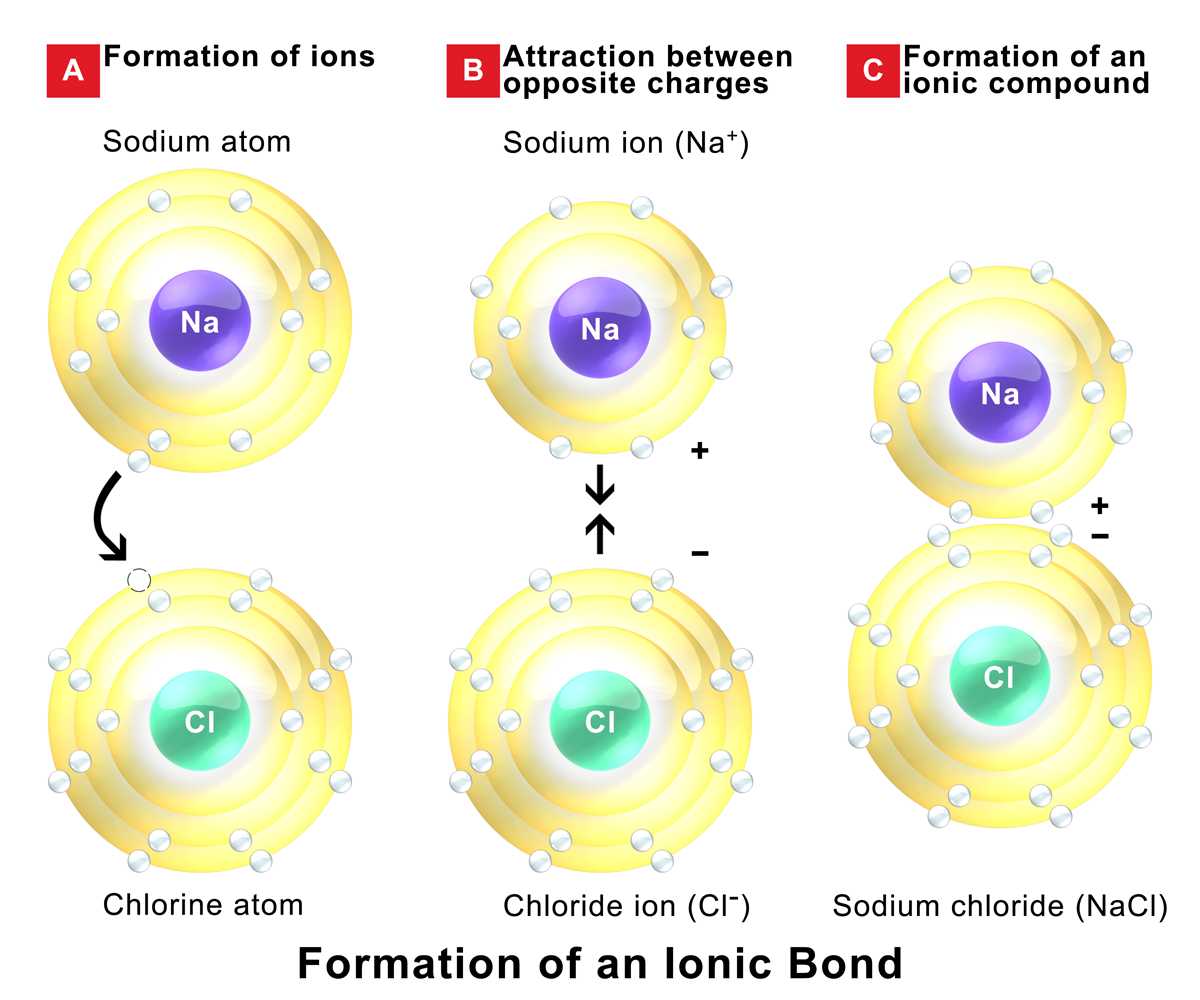
Ionic Bonds Form Between
Ionic Bonds Form Between - Some compounds contain both covalent and ionic bonds. In doing so, cations are formed. So how do you know what kind of bond an atom will make? [noun] a chemical bond formed between oppositely charged species because of their mutual electrostatic attraction. These oppositely charged ions attract each other to form ionic networks (or lattices ). Web bonds between two nonmetals are generally covalent; An atom of another element (usually nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accepting ele… Electron transfer produces negative ions called anions and positive ions. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: It is a type of chemical bond that generates two oppositely charged ions.
Electrostatic forces of attraction between. [noun] a chemical bond formed between oppositely charged species because of their mutual electrostatic attraction. An atom of another element (usually nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accepting ele… Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web ionic bond definition, the electrostatic bond between two ions formed through the transfer of one or more electrons. Web bonds between two nonmetals are generally covalent; Some compounds contain both covalent and ionic bonds. Web ionic bonds form when two or more ions come together and are held together by charge differences. Web condition for ionic bonding. The two elements or ions.
Web to illustrate further, consider the two major types of chemical bonds: For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Web ionic bond definition, the electrostatic bond between two ions formed through the transfer of one or more electrons. Covalent bonds and ionic bonds. In doing so, cations are formed. Electrostatic forces of attraction between. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Web forming an ionic bond the chemical bond that is formed between 2 2 atoms through the transfer of one or more electrons from the electropositive or metallic. Some compounds contain both covalent and ionic bonds. So how do you know what kind of bond an atom will make?
Ionic Bond Definition, Types, Properties & Examples
For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Electron transfer produces negative ions called anions and positive ions. So how do you know what kind of bond an atom will make? The following are the conditions for the formation of ionic bonding between chemical substances; Some compounds contain both covalent.
Ionic Properties
It is a type of chemical bond that generates two oppositely charged ions. Web condition for ionic bonding. Covalent bonds and ionic bonds. These oppositely charged ions attract each other to form ionic networks (or lattices ). Web bonds between two nonmetals are generally covalent;
Ionic Bond Definition, Types, Properties & Examples
Web ionic bonds form when two or more ions come together and are held together by charge differences. Some compounds contain both covalent and ionic bonds. For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. The oppositely charged ions are strongly attracted to. It is a type of chemical bond that.
Electronegativity Bond Scale Surfguppy Chemistry made easy for
Web ionic bonding is the complete transfer of valence electron(s) between atoms. Some compounds contain both covalent and ionic bonds. An atom of another element (usually nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accepting ele… The oppositely charged ions are strongly attracted to. [noun] a chemical bond formed between.
Examples of Ionic Bonds and Ionic Compounds
Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web bonds between two nonmetals are generally covalent; Web forming an ionic bond the chemical bond that is formed between 2 2 atoms through the transfer of one or more electrons from the electropositive.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
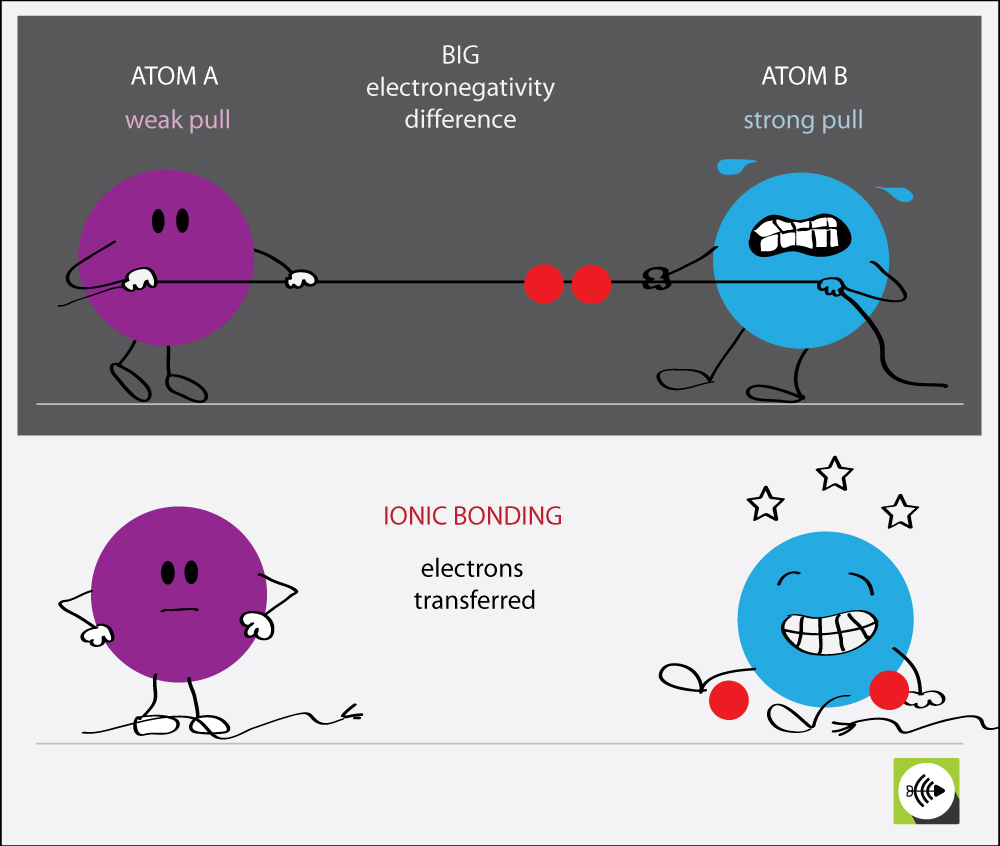
Web ionic bond definition, the electrostatic bond between two ions formed through the transfer of one or more electrons. The oppositely charged ions are strongly attracted to. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their electrons to achieve a stable electron configuration. Covalent bonds.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Covalent bonds and ionic bonds. Electrostatic forces of attraction between. For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. In covalent bonds, two atoms share pairs of electrons, while in ionic.
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Web ionic bonds are one of the two main types of chemical bonds. Web bonds between two nonmetals are generally covalent; In covalent bonds, two atoms share pairs of electrons, while in ionic. In doing so, cations are formed. Web ionic bonds usually occur between metal and nonmetal ions.
Chapter 2 Atoms, Molecules and Life Chemistry)
The oppositely charged ions are strongly attracted to. [noun] a chemical bond formed between oppositely charged species because of their mutual electrostatic attraction. An atom of another element (usually nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accepting ele… The following are the conditions for the formation of ionic bonding.
10 Notable Differences Between Ionic And Covalent Bonds Current
The oppositely charged ions are strongly attracted to. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), whose ionization energy is low, give some of their electrons to achieve a stable electron configuration. The following are the conditions for the formation of ionic bonding between chemical substances; It is a type of chemical bond.
Web Bonds Between Two Nonmetals Are Generally Covalent;
[noun] a chemical bond formed between oppositely charged species because of their mutual electrostatic attraction. Web to illustrate further, consider the two major types of chemical bonds: Bonding between a metal and a nonmetal is often ionic. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms.
Some Compounds Contain Both Covalent And Ionic Bonds.
Covalent bonds and ionic bonds. An atom of another element (usually nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accepting ele… Web ionic bonds are formed between cations and anions. Web ionic bond definition, the electrostatic bond between two ions formed through the transfer of one or more electrons.
Web Ionic Bonds Form When Two Or More Ions Come Together And Are Held Together By Charge Differences.
Electron transfer produces negative ions called anions and positive ions. Web ionic bonding is the complete transfer of valence electron(s) between atoms. These oppositely charged ions attract each other to form ionic networks (or lattices ). Web condition for ionic bonding.
It Is A Type Of Chemical Bond That Generates Two Oppositely Charged Ions.
Electrostatic forces of attraction between. Web forming an ionic bond the chemical bond that is formed between 2 2 atoms through the transfer of one or more electrons from the electropositive or metallic. Web ionic bonds usually occur between metal and nonmetal ions. In doing so, cations are formed.



:max_bytes(150000):strip_icc()/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
.PNG)