How Many Bonds Does Bromine Form
How Many Bonds Does Bromine Form - A colorless gas, it dissolves in. The chemical symbol for bromine is. Web bromine is a 35. Web bromine is a member of the halogen family of elements. Web how many covalent bonds will bromine and iodine form? Web posted sep 14, 2022. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet. They will form seven bonds along with all the other elements in that column on the periodic table. Web bromine will normally form one covalent bond.
Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet. A single bond and two double bonds. Web posted sep 14, 2022. Bromine is a halogen and has 7 valence electrons. Chemical element in the periodic table of elements. It has 35 protons and 35 electrons in the atomic structure. Web bromine will normally form one covalent bond. Web bromine will normally form one covalent bond. It can form 1, 2, or 3 bonds with other atoms. Molybdenum bromine oxygen potassium nitrogen how many single covalent bonds does each element.
Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Chemical element in the periodic table of elements. It has 35 protons and 35 electrons in the atomic structure. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. 6 which elements tend to form covalent. Bromine atoms have a strong. Modeling lonic and covalent bonds part 1: •br br submit your choice. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Its companions include fluorine, chlorine, and iodine.
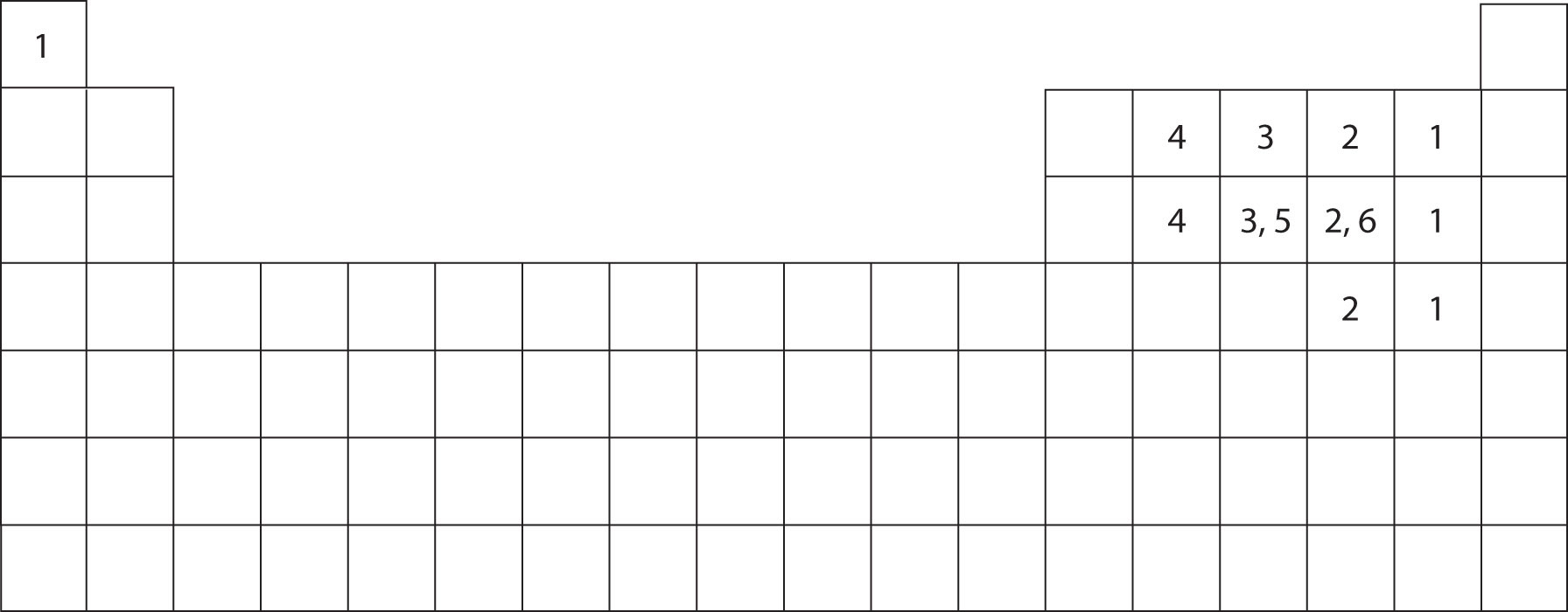
HONC 1234 ChemSimplified
Web bromine will normally form one covalent bond. Chemical element in the periodic table of elements. Web how many covalent bonds will bromine and iodine form? The maximum number of bonds. 6 which elements tend to form covalent.
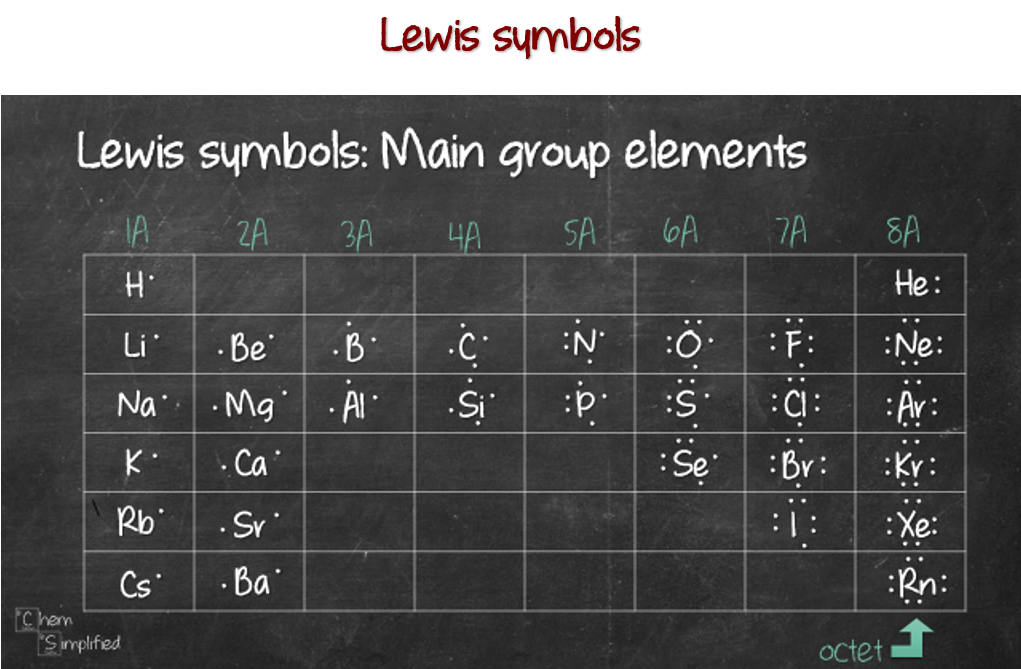
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram
Web the circles show how the valence electron shells are filled for both atoms. Modeling lonic and covalent bonds part 1: Like the other halogens, bromine has seven electrons in its outer. Chemical element in the periodic table of elements. Hydrogen bromide is the inorganic compound with the formula h br.
How Many Bonds Does Bromine Form? Answer]
It is the process of two or more atoms coming together. Web how many covalent bonds will bromine and iodine form? 0 how many bonds will a carbon atom form with four bromine atoms? The atomic number of bromine is 35, and the atomic weight is 79.904. The chemical symbol for bromine is.
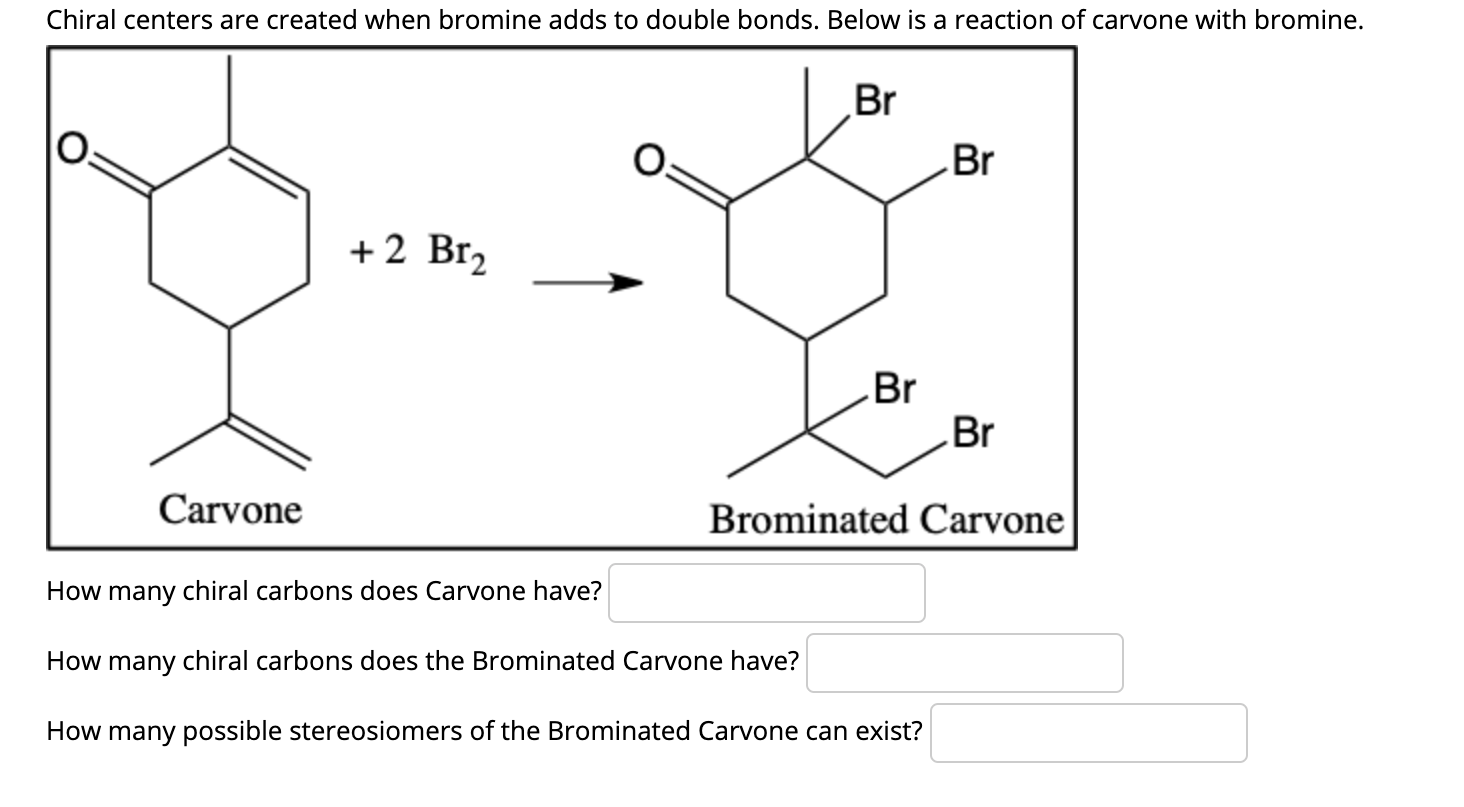
Solved Chiral centers are created when bromine adds to
The chemical symbol for bromine is. How many bonds can bromine make? Web the circles show how the valence electron shells are filled for both atoms. It is the process of two or more atoms coming together. Web bromine will normally form one covalent bond.
how many bonds does sulfur form
0 how many bonds will a carbon atom form with four bromine atoms? Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Bromine.
Bromine Protons Neutrons Electrons Electron Configuration
Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. 6 which elements tend to form covalent. •br br submit your choice. Web bromine is a 35. Bromine is a halogen and has 7 valence electrons.
How Many Bonds Does Nitrogen Form tour bous
Web how many covalent bonds will bromine and iodine form? Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next It has 35 protons and 35 electrons in the atomic structure. A colorless gas, it dissolves in. Its companions include.
Collins New GCSE Science Gateway B page 112
The atomic number of bromine is 35, and the atomic weight is 79.904. Two single bonds and a double bond. The maximum number of bonds. The chemical symbol for bromine is. Hydrogen bromide is the inorganic compound with the formula h br.
How to Predict number of bonds each element forms ChemSimplified
6 which elements tend to form covalent. The atomic number of bromine is 35, and the atomic weight is 79.904. Web how many bonds does bromine form? It is a hydrogen halide consisting of hydrogen and bromine. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence.
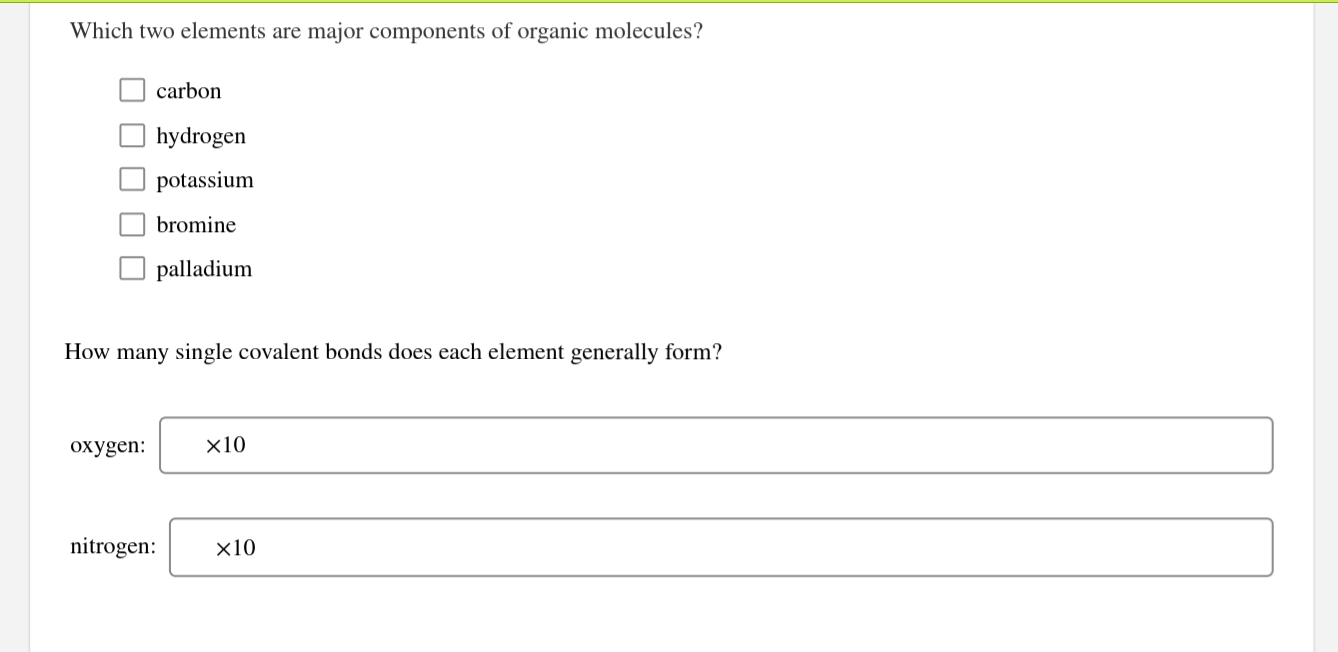
Solved Which two elements are major components of organic
Web it is a halogen and has an atomic symbol br. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. It is the process of two or more atoms coming together. Web bromine is a member of the halogen family of elements. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images.
Study Guides Chemistry 19 Cards To Name A Monatomic Anion Change The Suffix Of The Element's Name To The.
Chemical element in the periodic table of elements. Two single bonds and a double bond. A single bond and two double bonds. Which of the following situations meet the bonding requirement for carbon atoms.
Web Posted Sep 14, 2022.
Web how many covalent bonds will bromine and iodine form? The chemical symbol for bromine is. Hydrogen bromide is the inorganic compound with the formula h br. It is a hydrogen halide consisting of hydrogen and bromine.
Web The Circles Show How The Valence Electron Shells Are Filled For Both Atoms.
It has 35 protons and 35 electrons in the atomic structure. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Like the other halogens, bromine has seven electrons in its outer.
Bromine Atoms Have A Strong.
A colorless gas, it dissolves in. The maximum number of bonds. Bromine is a halogen and has 7 valence electrons. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next

![How Many Bonds Does Bromine Form? Answer]](https://images.pexels.com/photos/6373491/pexels-photo-6373491.jpeg?auto=compress&cs=tinysrgb&w=1200)