How Many Bonds Can Iodine Form
How Many Bonds Can Iodine Form - Since iodine (i) is below period 3 on the periodic table it can hold more than 8 electrons. Web how many covalent bonds does iodine form? Web trending can iodine form double bonds? Web iodine combines readily with most metals and some nonmetals to form iodides; Web to determine the number of single bonds can form iodine we need to determine the number valency of an iodine atom.valence electrons are the outer shell. For example, silver and aluminum are easily converted into their respective iodides, and. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Consider a compound that forms between the elements iodine and bromine, iodine monobromide, ibr. Web this supports a mechanism whereby i 2 first dissociates into 2 iodine atoms, which each attach themselves to a side of an h 2 molecule and break the h−h bond: Web iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation i 2 + cl 2 → 2 icl.
Web how many covalent bonds does iodine form? Therefore, carbon molecules share their 4 valence electrons through single,. Web trending can iodine form double bonds? Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web how many covalent bonds does iodine form? When chlorine gas is passed through iodine. 2 is i2 a single or double bond? Consider a compound that forms between the elements iodine and bromine, iodine monobromide, ibr. The i 2 molecules tend to interact via the weak van der. For example, silver and aluminum are easily converted into their respective iodides, and.
Web how many covalent bonds does iodine form? H 2 + i 2 +. Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause. Web this supports a mechanism whereby i 2 first dissociates into 2 iodine atoms, which each attach themselves to a side of an h 2 molecule and break the h−h bond: The i 2 molecules tend to interact via the weak van der. Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause. For example, silver and aluminum are easily converted into their respective iodides, and. Web iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation i 2 + cl 2 → 2 icl. Web trending can iodine form double bonds? 2 is i2 a single or double bond?
4.1 Covalent Bonds The Basics of General, Organic, and Biological
When chlorine gas is passed through iodine. Since iodine (i) is below period 3 on the periodic table it can hold more than 8 electrons. Web illustrate covalent bond formation using lewis diagrams. Web iodine combines readily with most metals and some nonmetals to form iodides; Web iodine monochloride is produced simply by combining the halogens in a 1:1 molar.
Is SiO2 Ionic or Covalent? Techiescientist
Web trending can iodine form double bonds? Therefore, carbon molecules share their 4 valence electrons through single,. Web this supports a mechanism whereby i 2 first dissociates into 2 iodine atoms, which each attach themselves to a side of an h 2 molecule and break the h−h bond: Since iodine (i) is below period 3 on the periodic table it.
Atomic Structure And Chemical Bonds Worksheet Answer Key Free Nude
Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. 2 is i2 a single or double bond? Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause. Since iodine (i) is below period 3 on the periodic table it can hold more than 8 electrons. Web illustrate covalent bond formation using.
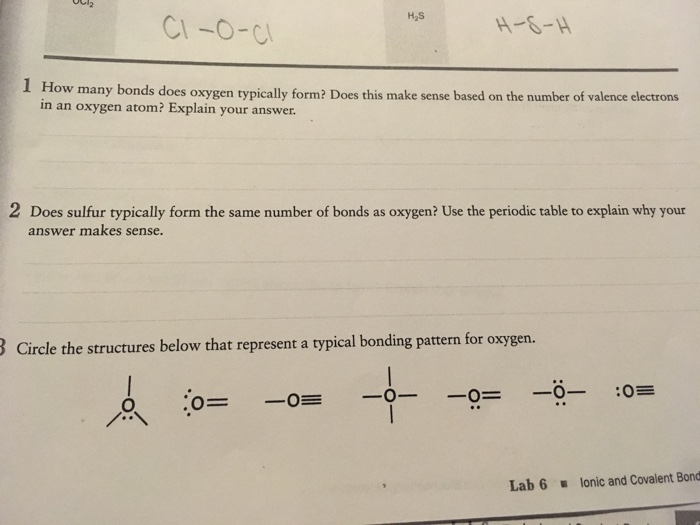
Solved H2S CIOC HSH 1 How many bonds does oxygen
Consider a compound that forms between the elements iodine and bromine, iodine monobromide, ibr. Therefore, carbon molecules share their 4 valence electrons through single,. Web iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation i 2 + cl 2 → 2 icl. Web iodine combines readily with most metals and some.
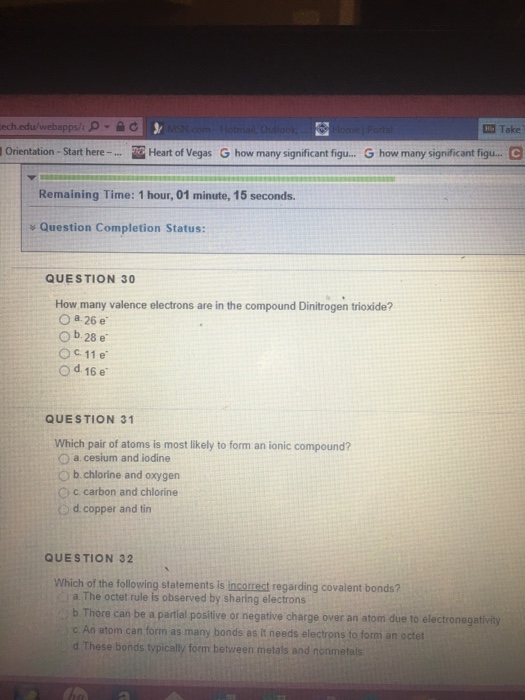
Solved How many valence electrons are in the compound
Web this supports a mechanism whereby i 2 first dissociates into 2 iodine atoms, which each attach themselves to a side of an h 2 molecule and break the h−h bond: Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Author june 20, 2020 table of contents [ hide] 1 can iodine form double bonds?.
Iodine Facts and Health Benefits Nutrition
Therefore, carbon molecules share their 4 valence electrons through single,. 2 is i2 a single or double bond? Web iodine combines readily with most metals and some nonmetals to form iodides; H 2 + i 2 +. Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web illustrate covalent bond formation using lewis diagrams. Consider a compound that forms between the elements iodine and bromine, iodine monobromide, ibr. Web this supports a mechanism whereby i 2 first dissociates into 2 iodine atoms, which each attach themselves to a side of an h 2 molecule and break the h−h bond: Iodine typically forms only 1 covalent bond,.
PPT Chemical Bonding PowerPoint Presentation, free download ID1989892
Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. For example, silver and aluminum are easily converted into their respective iodides, and. Web iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation i 2 + cl 2 → 2 icl. Web illustrate covalent bond formation using.
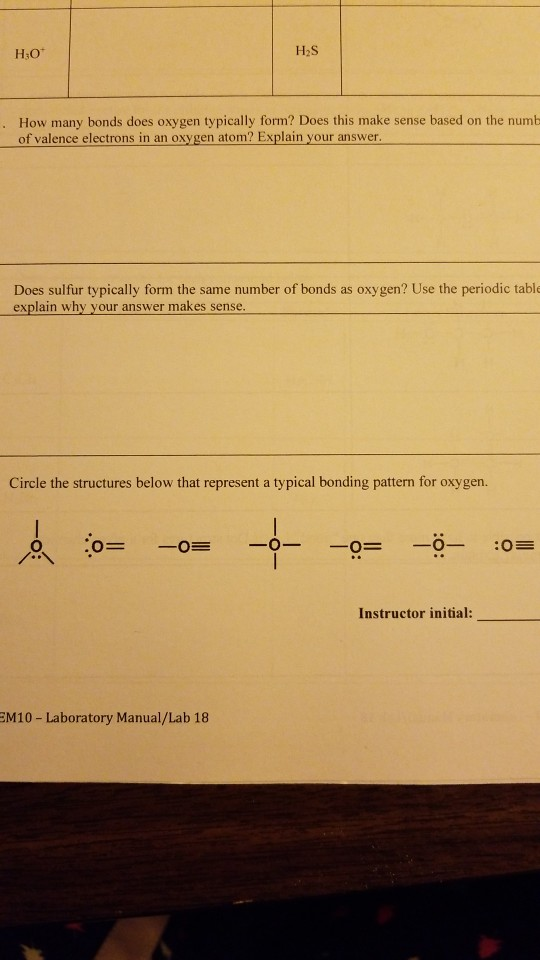
Solved HO How many bonds does oxygen typically form? Does
Web how many covalent bonds does iodine form? Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause. 2 is i2 a single or double bond? Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web to determine the number of single bonds can form iodine we need.
Solved How many covalent bonds can an element of Group VA
Consider a compound that forms between the elements iodine and bromine, iodine monobromide, ibr. Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause. For example, silver and aluminum are easily converted into their respective iodides, and. Web to determine the number of single bonds can form iodine we need to determine the.
Web This Supports A Mechanism Whereby I 2 First Dissociates Into 2 Iodine Atoms, Which Each Attach Themselves To A Side Of An H 2 Molecule And Break The H−H Bond:
Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause. Web trending can iodine form double bonds? Web to determine the number of single bonds can form iodine we need to determine the number valency of an iodine atom.valence electrons are the outer shell. Since iodine (i) is below period 3 on the periodic table it can hold more than 8 electrons.
When Chlorine Gas Is Passed Through Iodine.
For example, silver and aluminum are easily converted into their respective iodides, and. The i 2 molecules tend to interact via the weak van der. Web iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation i 2 + cl 2 → 2 icl. 2 is i2 a single or double bond?
Sources, Facts, Uses, Scarcity (Sri), Podcasts, Alchemical Symbols, Videos And Images.
Author june 20, 2020 table of contents [ hide] 1 can iodine form double bonds? Therefore, carbon molecules share their 4 valence electrons through single,. Iodine typically forms only 1 covalent bond, but sometimes elements like fluorine, chlorine, and oxygen can cause. Web iodine combines readily with most metals and some nonmetals to form iodides;
Web Iodate Ion Contains One Iodine And Three Oxygen Atoms.
Web illustrate covalent bond formation using lewis diagrams. Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web how many covalent bonds does iodine form? Consider a compound that forms between the elements iodine and bromine, iodine monobromide, ibr.