Fda Form 483 Response Time
Fda Form 483 Response Time - Web this response must be submitted within 15 business days regardless of the number of observations, as of september 2009. [8] [9] while a response is not compulsory, a good. Web the long description is entered into the fda form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered, and the citations. Web to document and clarify our thought processes and positions at that time. Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). Web your fda 483 response is required in less than 15 business days. When drafting your response, it’s best to follow a standard outline. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. Web how to respond to fda form 483s and warning letters.
Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary understand significance of observations relating to. Web when you receive an fda form 483, you must respond within 15 business days. Web this response must be submitted within 15 business days regardless of the number of observations, as of september 2009. Web how to respond to fda form 483s and warning letters. That said, requesting a 483 can be costly and may take a lot of time. Many medical device manufacturers receive fda warning letters due to lack of preparation for the fda. However, to make sure that your response is timely, it's best to respond within 15. Web fda 483 observations are listed on fda’s inspectional observations form when in the investigator’s judgment, conditions or practices observed would indicate that any food,. Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). When drafting your response, it’s best to follow a standard outline.
Web the long description is entered into the fda form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered, and the citations. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. Web aform fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observedconditions that in their judgment may constitute violations of. [8] [9] while a response is not compulsory, a good. The fda must scrub/redact any potentially. That said, requesting a 483 can be costly and may take a lot of time. Web the fda is interested in the corrective actions you intend to take to fix the situation that led to the warning letter or form 483 — not justifications. Web structuring your fda 483 response. That outline has 3 parts: The fda has always involuntarily required a medical device firm, or any firm under fda.
FDA Form 483 Observations and Warning Letters What’s the Difference?
That outline has 3 parts: Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web this response must be submitted within 15 business days regardless of the number of observations, as of september 2009. The fda has always involuntarily required a medical device firm, or any firm under fda. However, to make sure.
PolarityTE FDA Form 483
When drafting your response, it’s best to follow a standard outline. Web the long description is entered into the fda form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered, and the citations. Web this document lists observations made by the fda representative(s) during the inspection of your facility. [8] [9] while a response.
LOGO
Web the fda is interested in the corrective actions you intend to take to fix the situation that led to the warning letter or form 483 — not justifications. Web how to respond to fda form 483s and warning letters. Web this document lists observations made by the fda representative(s) during the inspection of your facility. The fda has always.
How to Respond FDA Form 483 and Warning Letters Know its differences
Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). Web structuring your fda 483 response. Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary understand significance of observations relating to. Web the.
With 4.3 billion pending sale, Akorn faces anonymous misconduct
Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary understand significance of observations relating to. The fda must scrub/redact any potentially. Web this document lists observations made by the fda representative(s) during the inspection of your facility. That said, requesting a 483 can be costly and may.
5 Common Mistakes to Avoid in Your FDA 483 Response
When drafting your response, it’s best to follow a standard outline. Web structuring your fda 483 response. Web when you receive an fda form 483, you must respond within 15 business days. Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary understand significance of observations relating to..
FDA Form483 The SUPPLEMENT Page 6
Web how to respond to fda form 483s and warning letters. Web aform fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observedconditions that in their judgment may constitute violations of. Many medical device manufacturers receive fda warning letters due to lack of preparation for the fda. That said, requesting a 483.
With 4.3 billion pending sale, Akorn faces anonymous misconduct
However, to make sure that your response is timely, it's best to respond within 15. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web how to respond to fda form 483s and warning letters. Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial.
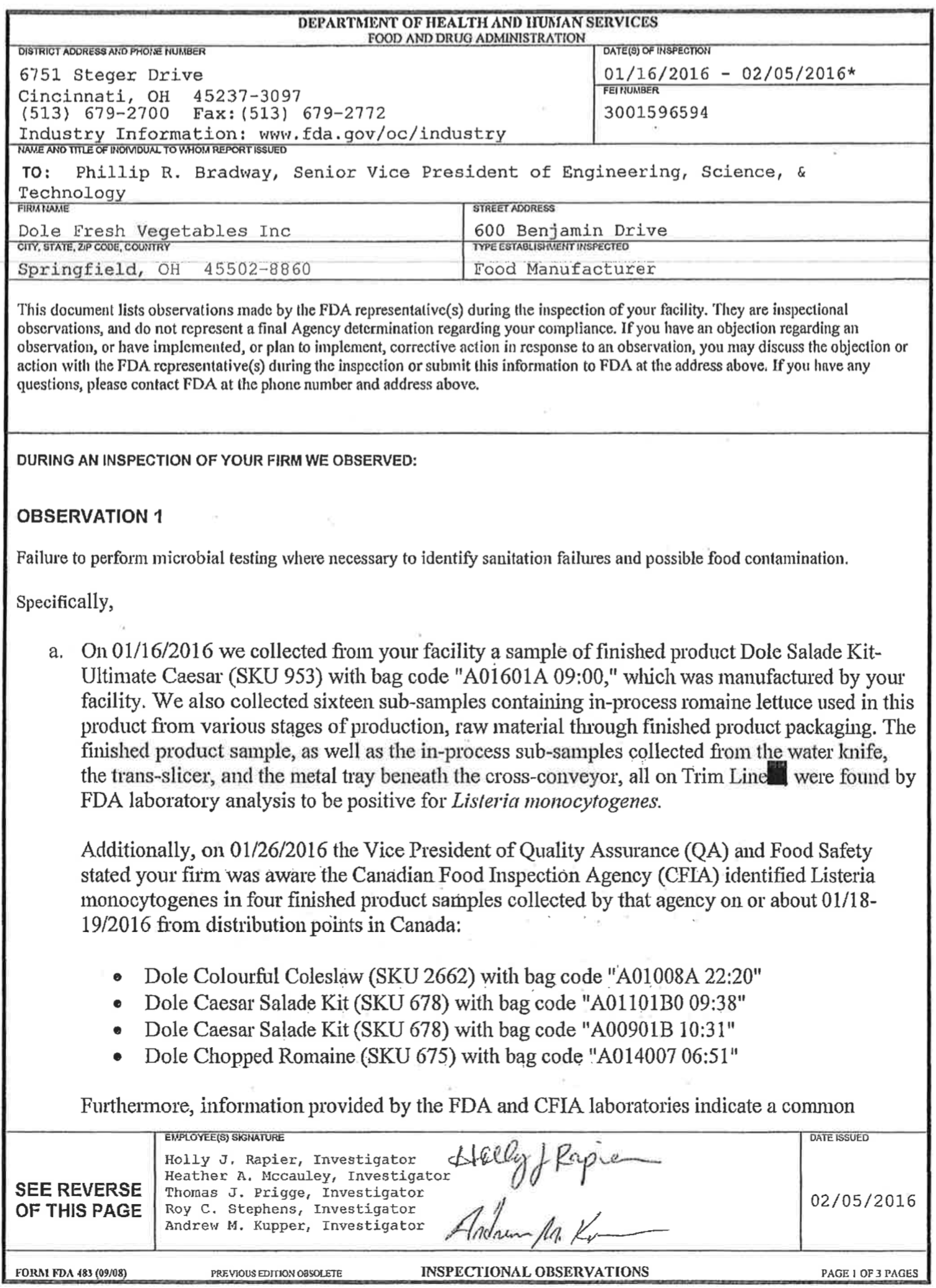
Dole’s FDA 483 Window into Lettuce Production Marler Blog
Web this document lists observations made by the fda representative(s) during the inspection of your facility. That outline has 3 parts: Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. In our responses to the fda form 483 observations, eli lilly and.
2015 FDA Form 483 Observations
That said, requesting a 483 can be costly and may take a lot of time. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. [8] [9] while a response is not compulsory, a good. Web any 483 can be requested by anyone..
The Fda Must Scrub/Redact Any Potentially.
Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). Web how to respond to fda form 483s and warning letters. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web to document and clarify our thought processes and positions at that time.
That Said, Requesting A 483 Can Be Costly And May Take A Lot Of Time.
You are not required by law. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. Web the fda is interested in the corrective actions you intend to take to fix the situation that led to the warning letter or form 483 — not justifications. When drafting your response, it’s best to follow a standard outline.
Web When You Receive An Fda Form 483, You Must Respond Within 15 Business Days.
Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary understand significance of observations relating to. Web fda 483 observations are listed on fda’s inspectional observations form when in the investigator’s judgment, conditions or practices observed would indicate that any food,. Web any 483 can be requested by anyone. Web aform fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observedconditions that in their judgment may constitute violations of.
In Our Responses To The Fda Form 483 Observations, Eli Lilly And Company Commits To Change The.
Web structuring your fda 483 response. [8] [9] while a response is not compulsory, a good. Many medical device manufacturers receive fda warning letters due to lack of preparation for the fda. However, to make sure that your response is timely, it's best to respond within 15.