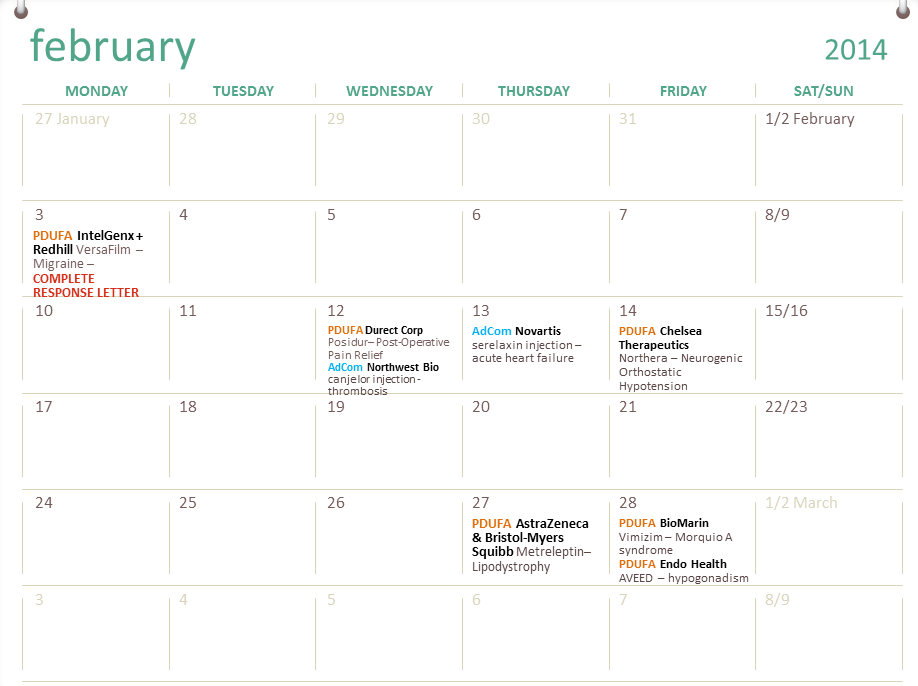
Fda Calendar For Drug Approval
Fda Calendar For Drug Approval - Innovative drugs often mean new treatment options for patients. Web cder drug and biologic approvals for calendar year 2022; Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Web this week's drug approvals. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Cder drug and biologic approvals for calendar year. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web 24 rows novel drug approvals for 2023.
Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Innovative drugs often mean new treatment options for patients. Resources for information | approved drugs. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Web this week's drug approvals. Cder drug and biologic approvals for calendar year. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web 24 rows novel drug approvals for 2023. Web cder drug and biologic approvals for calendar year 2022;
Web 24 rows novel drug approvals for 2023. Resources for information | approved drugs. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Web this week's drug approvals. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Cder drug and biologic approvals for calendar year. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Innovative drugs often mean new treatment options for patients. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital.
FDA Calendar FDA Tracker
Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Cder highlights key web sites. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web cder drug and biologic approvals for calendar year 2022; Cder drug and biologic approvals for calendar year.
FDA Calendar FDA Tracker
Resources for information | approved drugs. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Cder drug and biologic approvals for calendar year. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web the fda’s 2024 pdufa calendar is far from complete, but.
UPDATED The Global Pharmaceutical Serialisation Calendar 2014 2018
Cder highlights key web sites. Cder drug and biologic approvals for calendar year. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Human drugs and therapeutic biologicals (proteins and other products derived from.
FDA Calendar FDA Tracker
Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Web the fda’s 2024 pdufa calendar is far from complete, but.
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Cder highlights key web sites. Cder drug and biologic approvals for calendar year. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web cder drug and biologic approvals for calendar year 2022;
The Most Important New Drug Of 2014
Web this week's drug approvals. Web an fda calendar typically displays information about the expected timeline for a particular drug approval or. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Innovative drugs.
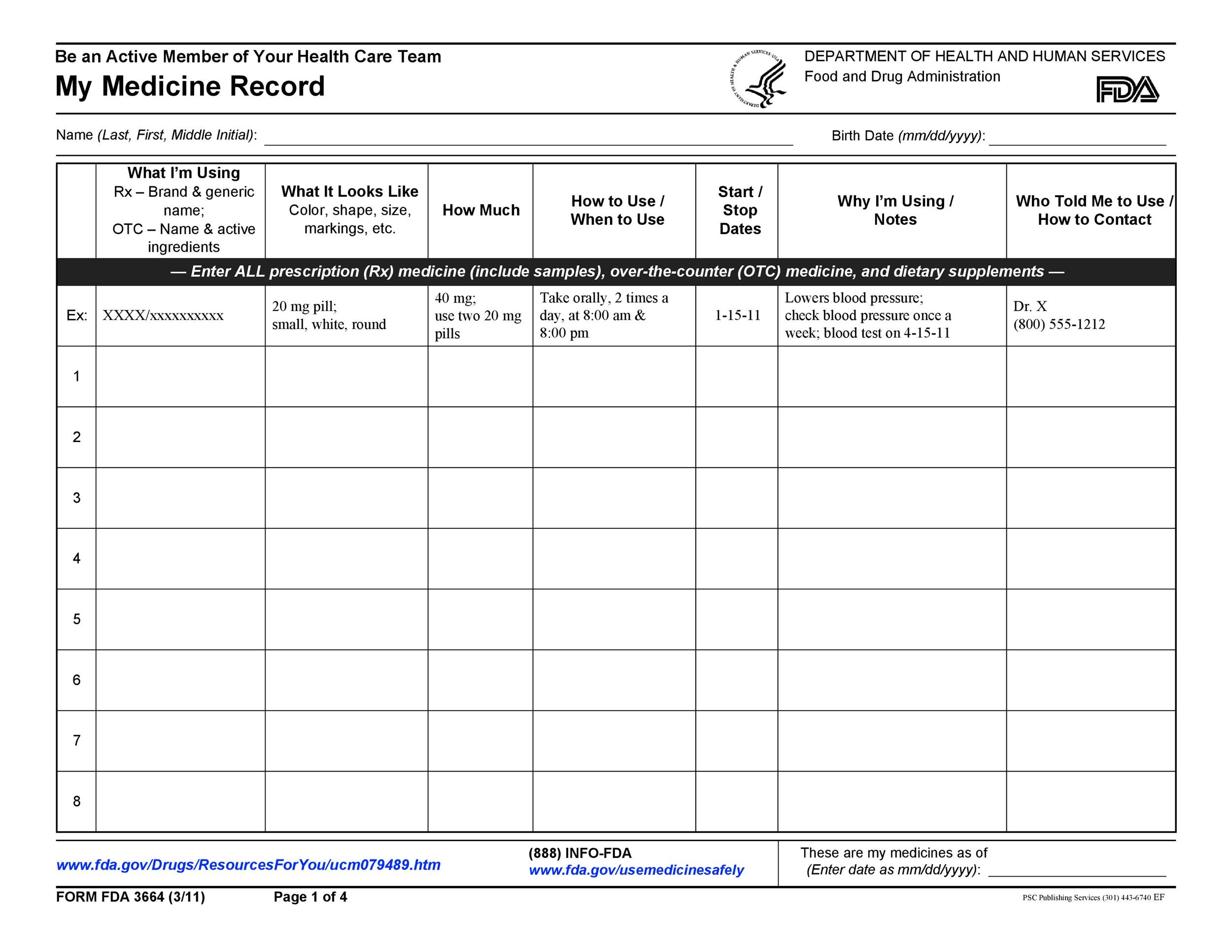
40 Great Medication Schedule Templates (+Medication Calendars)
Web 24 rows novel drug approvals for 2023. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Cder drug and biologic approvals for calendar year. Resources for information | approved drugs. Web cder drug and biologic approvals for calendar year 2022;
FDA Calendar FDA Tracker
Innovative drugs often mean new treatment options for patients. Resources for information | approved drugs. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web cder drug and biologic approvals for calendar year 2022;
FDA Calendar FDA Tracker
Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Resources for information | approved drugs. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for. Innovative drugs often.
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
Cder highlights key web sites. Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital. Web 24 rows novel drug approvals for 2023. Web this week's drug approvals.
Web Cder Drug And Biologic Approvals For Calendar Year 2022;
Web this week's drug approvals. Cder drug and biologic approvals for calendar year. Resources for information | approved drugs. Human drugs and therapeutic biologicals (proteins and other products derived from living sources used for.
Web An Fda Calendar Typically Displays Information About The Expected Timeline For A Particular Drug Approval Or.
Web 24 rows novel drug approvals for 2023. Innovative drugs often mean new treatment options for patients. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching,. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital.
Cder Highlights Key Web Sites.
Web below is a listing of new molecular entities and new therapeutic biological products that cder approved in.