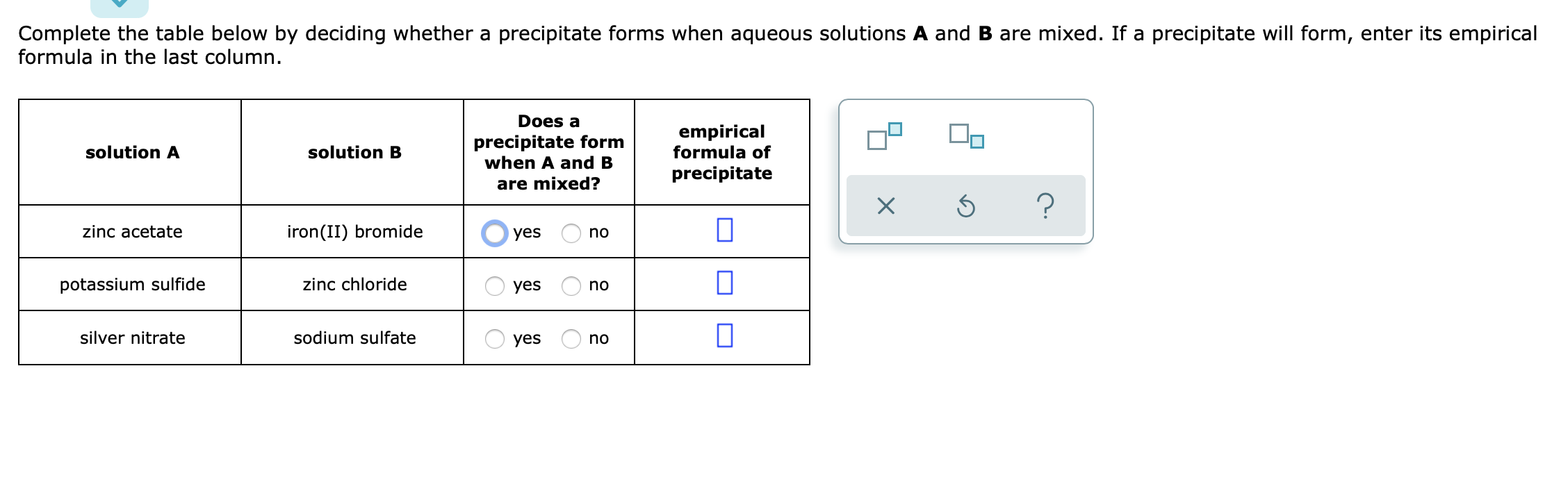
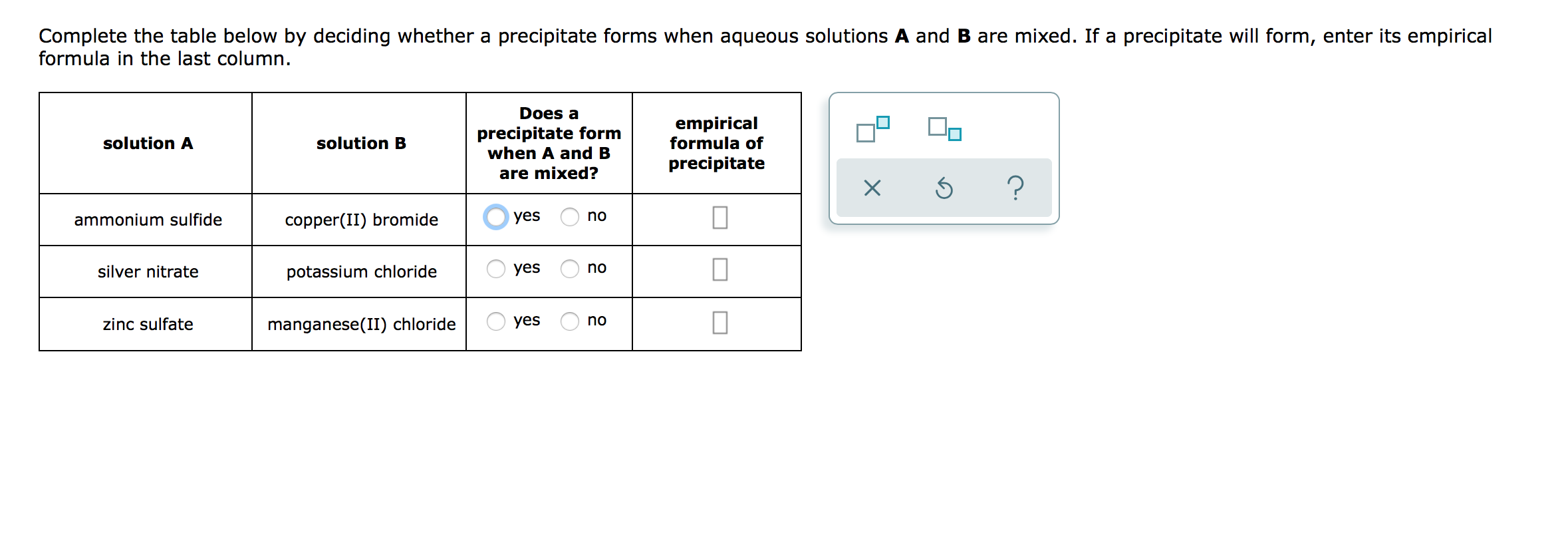
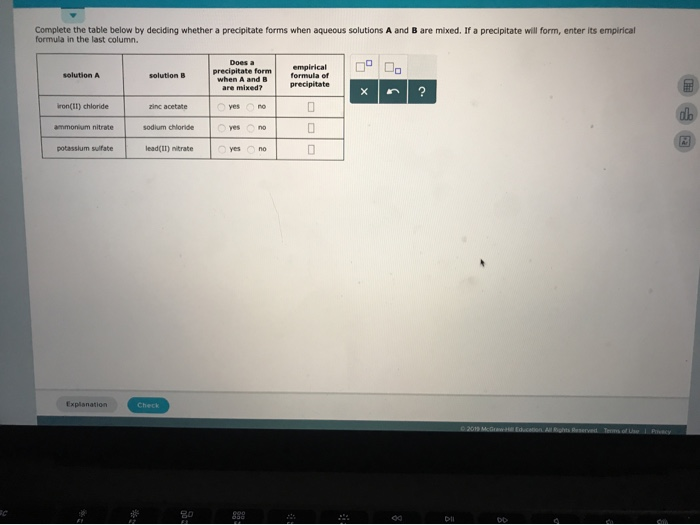
Does A Precipitate Form When A And B Are Mixed
Does A Precipitate Form When A And B Are Mixed - Empirical formula of precipitate silver. 100% (1 rating) transcribed image text: All right, so this question is asking us: Web science chemistry does a precipitate form when a and b empirical formula of solution a solution b precipitate are mixed? Solution a solution b does a precipitate form when a and b are mixed? Does a precipitate form when. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Empirical formula of precipitate х 5 ? Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Empirical formula of precipitate solution a solution b lead (ii) nitrate potassium chloride yes no sodium acetate.
Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web in the first case; Empirical formula of precipitate solution a solution b lead (ii) nitrate potassium chloride yes no sodium acetate. Empirical formula of precipitate solution a solution b sodium hydroxide iron (ii). Zinc sulfate iron (ii) iodide o yes no iron (ii) sulfate. Solution a solution b does a precipitate form when a and b are mixed? Does a precipitate form when a and b are mixed? All right, so this question is asking us: Iron (iii) bromide sodium hydroxide o yes o no zinc acetate iron. Does a precipitate form when a and b are mixed?
Solution a solution b does a precipitate form when a and b are mixed? Copper (ii) chloride potassium hydroxide yes no. Solution a solution b does a precipitate form when a and b are mixed? Zinc sulfate iron (ii) iodide o yes no iron (ii) sulfate. Formula in the last column. Web does a precipitate form when a and b are mixed? Web if a precipitate will form, enter its empirical formula in the last column does a precipitate form when a and b are mixed? Web complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Empirical formula of precipitate zinc chloride potassium hydroxide oyes no silver nitrate. Empirical formula of precipitate potassium hydroxide iron (iii) chloride o.
Solved Complete the table below by deciding whether a
Empirical formula of precipitate solution a solution b lead (ii) nitrate potassium chloride yes no sodium acetate. Does a precipitate form when a and b are mixed? Web if a precipitate will form, enter its empirical formula in the last column does a precipitate form when a and b are mixed? Formula in the last column. If a precipitate will.
Dependable iron ii chloride and zinc acetate precipitate in U.K
Complete the table below by deciding whether a precipitate. Solution a solution b does a precipitate form when a and b are mixed? All right, so this question is asking us: Whether or not such a. Solution a solution b does a precipitate form when a and b are mixed?
Solved Complete the table below by deciding whether a
Whether or not such a. If a precipitate will form, enter it’s empirical formula in the. When we mix solution a which is an ionic compound and solution b, which is an ionic compound, are we going to form a. Does a precipitate form when. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and.
Complete the table below by deciding wheth... Organic Chemistry
Solution a solution b does a precipitate form when a and b are mixed? Empirical formula of precipitate zinc chloride potassium hydroxide oyes no silver nitrate. Solution a solution b does a precipitate form when a and b are mixed? Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Empirical formula.
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form
Web if a precipitate will form, enter its empirical formula in the last column does a precipitate form when a and b are mixed? Web chemistry questions and answers. Web chemistry questions and answers. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Web does a precipitate form when a and.
Precipitate Definition and Example in Chemistry
Web chemistry questions and answers. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Solution a solution b does a precipitate form when a and b are mixed? Does a precipitate form when. Empirical formula of precipitate potassium hydroxide iron (iii) chloride o.
Complete the table below by deciding whet... Physical Chemistry
Copper (ii) chloride potassium hydroxide yes no. Web complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Web does a precipitate form when a and b empirical formula of solution a solution b.
Solved Complete the table below by deciding whether a
Does a precipitate form when a and b are mixed? Solution a solution b does a precipitate form when a and b are mixed? Empirical formula of precipitate solution a solution b. Web complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Whether or not such a.
Complete the table below by deciding whet... Physical Chemistry
Empirical formula of precipitate zinc chloride potassium hydroxide oyes no silver nitrate. Empirical formula of precipitate solution a solution b. Web does a precipitate form when a and b empirical formula of solution a solution b precipitate are mixed? Copper (ii) chloride potassium hydroxide yes no. Solution a solution b does a precipitate form when a and b are mixed?
Solved Complete the table below by deciding whether a
Whether or not such a. Copper (ii) chloride potassium hydroxide yes no. Empirical formula of precipitate х 5 ? Web complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed.
Solution A Solution B Does A Precipitate Form When A And B Are Mixed?
Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. Complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. 100% (1 rating) transcribed image text: Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.
Solution A Solution B Does A Precipitate Form When A And B Are Mixed?
Empirical formula of precipitate potassium hydroxide iron (iii) chloride o. Empirical formula of precipitate silver. Empirical formula of precipitate solution a solution b. Does a precipitate form when.
Web Does A Precipitate Form When A And B Empirical Formula Of Solution A Solution B Precipitate Are Mixed?
If a precipitate will form, enter it’s empirical formula in the. If a precipitate will form, enter its empirical formula in the last. Web in the first case; Copper (ii) chloride potassium hydroxide yes no.
Complete The Table Below By Deciding Whether A Precipitate Forms When Aqueous Solutions A And B Are Mixed.
Empirical formula of precipitate zinc chloride potassium hydroxide oyes no silver nitrate. All right, so this question is asking us: Web chemistry questions and answers. Formula in the last column.