Chemistry Chapter 3 Review Answers
Chemistry Chapter 3 Review Answers - 16.4 potential, free energy, and equilibrium; Web study with quizlet and memorize flashcards containing terms like before there were chemists, _____ were studying matter. Web together by chemical bonds. Web the two masses have the same numerical value, but the units are different: Carbon dioxide and water are two examples, but answers will vary. Focus on this content, but make sure to review class notes,. Step 2 of 3 (or) step 3 of 3 Solving the rest of the problem: A) heterogeneous mixture b) solution (solid, of copper & zinc) c) solution. They developed _____ and _____ for working with chemicals., lavoisier helped make chemistry.
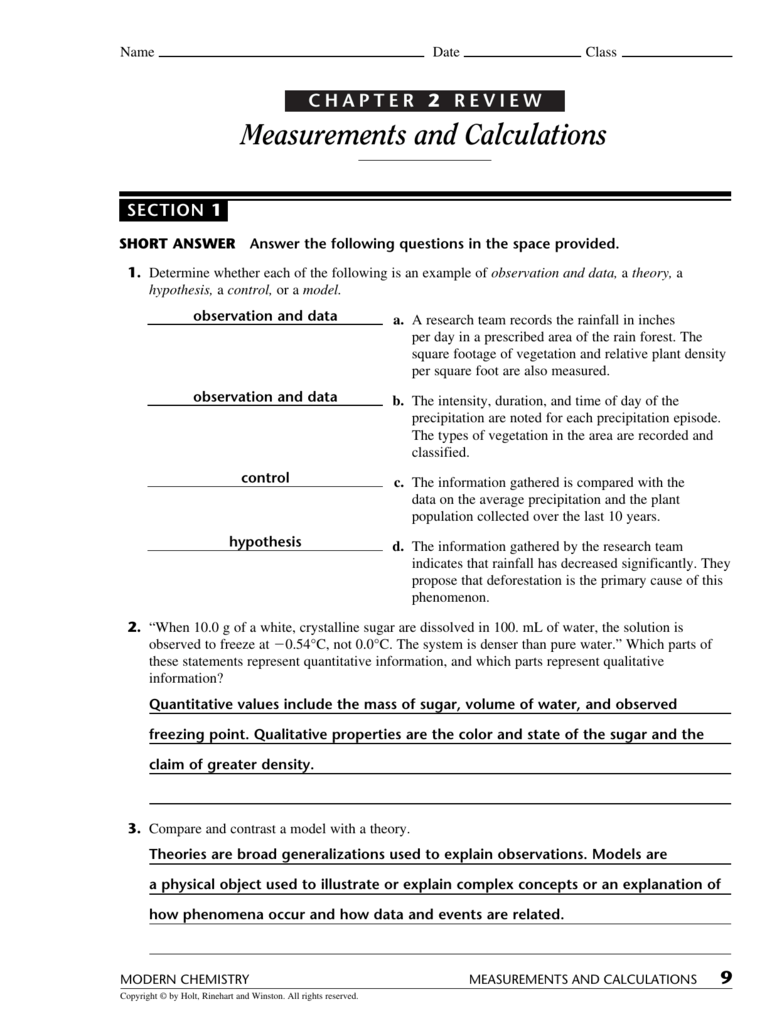
They developed _____ and _____ for working with chemicals., lavoisier helped make chemistry. 16.4 potential, free energy, and equilibrium; With expert solutions for thousands of practice problems, you. Web 1 / 9 law of conservation of mass, law of definite proportions, and law of multiple proportions click the card to flip 👆 flashcards learn test match created by dellageorge1331 terms in this set (9) what are the three branches of dalton's theory? Chapter 3 review 5.0 (1 review) term 1 / 25 law of conservation of mass click the card to flip 👆 definition 1 / 25 mass is neither created nor destroyed during ordinary chemical reactions or physical. He information for the chapter 3. Web exocytosis neurotransmitter molecules are often stored in synaptic ____________ near the presynaptic membrane. Carbon dioxide and water are two examples, but answers will vary. Solving the rest of the problem: This might also be a good time to read the chapter.
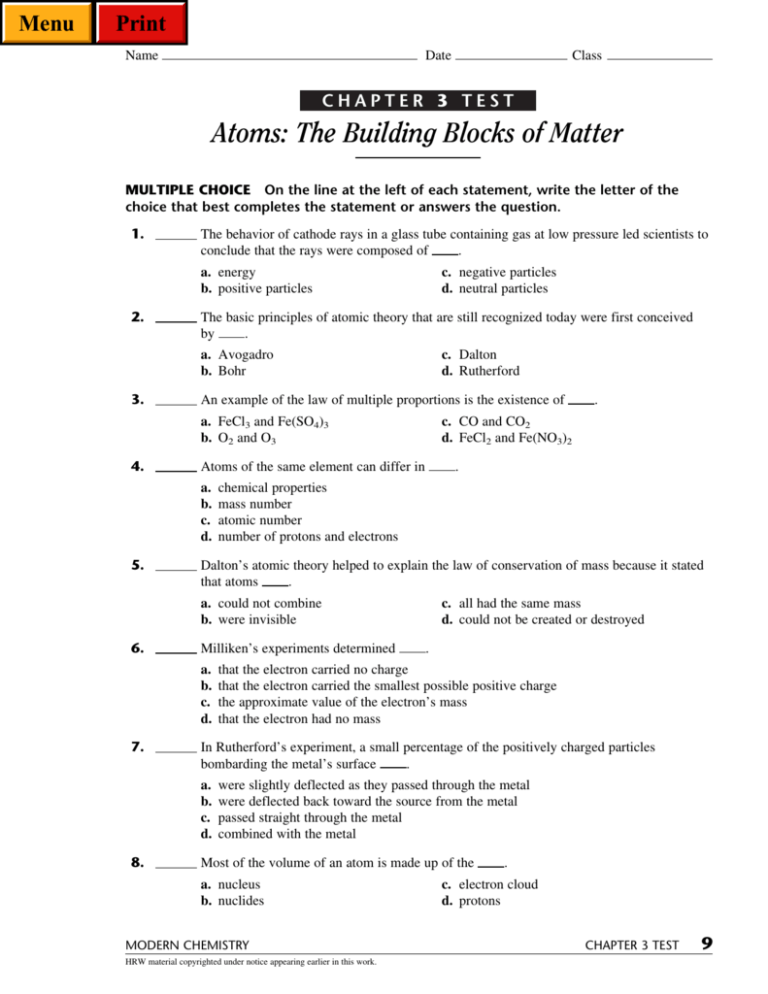
Rutherford’s experiment involved shooting a beam of particles at a thin sheet of metal foil. Focus on this content, but make sure to review class notes,. 16.4 potential, free energy, and equilibrium; The value of amu is given by as follows: Web exocytosis neurotransmitter molecules are often stored in synaptic ____________ near the presynaptic membrane. 5) in chemical reactions, atoms are. Law of conservation of mass, law of definite proportions, and law. He information for the chapter 3. You can test your readiness to proceed by answering the review. Step 2 of 3 (or) step 3 of 3
Holt Rinehart And Winston History Worksheet Answers Nidecmege
Web these are the answers to the honors chemistry midyear exam review chapter 3 questions. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The presentation of information in this chapter assumes that you can already perform. 16.3 electrode and cell potentials; Web chemistry chapter 3 review questions flashcards | quizlet.
StirlingIona
Vesicles together the dendrites and cell body are referred to as the ___________ area of a. Web our resource for modern chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. Web chemistry chapter 3 review questions flashcards | quizlet. Web together by chemical bonds. Web the two masses have.
modern chemistry chapter 1 test
Simply, it can be represented as amu. Chapter 3 review 5.0 (1 review) term 1 / 25 law of conservation of mass click the card to flip 👆 definition 1 / 25 mass is neither created nor destroyed during ordinary chemical reactions or physical. Objectives, which precede the review. Boils at 100°c and freezes at 0°c 3. This might also.
7 Best Images of Holt Science Worksheet Answers Photosynthesis and
Web 16.1 review of redox chemistry; Present in all atoms and elements 2. Web chemistry chapter 3 review questions flashcards | quizlet. 16.5 batteries and fuel cells; 16.3 electrode and cell potentials;
Chapter3 reviewanswers
16.5 batteries and fuel cells; 5) in chemical reactions, atoms are. This might also be a good time to read the chapter. Web 1 / 9 law of conservation of mass, law of definite proportions, and law of multiple proportions click the card to flip 👆 flashcards learn test match created by dellageorge1331 terms in this set (9) what are.
Chapter 8 Modern Chemistry Review Answers __HOT__
The value of amu is given by as follows: Objectives, which precede the review. Questions at the end of the chapter. The following pages contain the bulk (but not all) of t. Simply, it can be represented as amu.
Section 3.2 review key
Web 4 chapter 3 stoichiometry. Carbon dioxide and water are two examples, but answers will vary. The value of amu is given by as follows: The study of change 1. The molecular mass is the mass of 1 molecule while the molar mass is the mass of 6.022 × 10 23 molecules.
Mastering chemistry homework answers chapter 3
They developed _____ and _____ for working with chemicals., lavoisier helped make chemistry. Web together by chemical bonds. Vesicles together the dendrites and cell body are referred to as the ___________ area of a. Objectives, which precede the review. Note that if the calculated amount of o 2 was greater than 32 g, then we would have deduced that o.
Chemistry Chapter 3 Lesson 5 YouTube
The molecular mass is the mass of 1 molecule while the molar mass is the mass of 6.022 × 10 23 molecules. This might also be a good time to read the chapter. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Questions at the end of the chapter. 16.4 potential, free energy, and equilibrium;
Bestseller Chemistry Chapter 10 Section 3 Review Answers
Mass and charge never change 3… Web exocytosis neurotransmitter molecules are often stored in synaptic ____________ near the presynaptic membrane. Solving the rest of the problem: Colorless, odorless, and tasteless 2. They developed _____ and _____ for working with chemicals., lavoisier helped make chemistry.
Questions At The End Of The Chapter.
This might also be a good time to read the chapter. Simply, it can be represented as amu. Start studying chemistry chapter 3 review questions. Web 3) atoms of different elements differ in their physical and chemical properties.
They Developed _____ And _____ For Working With Chemicals., Lavoisier Helped Make Chemistry.
The molecular mass is the mass of 1 molecule while the molar mass is the mass of 6.022 × 10 23 molecules. Chapter 3 review 5.0 (1 review) term 1 / 25 law of conservation of mass click the card to flip 👆 definition 1 / 25 mass is neither created nor destroyed during ordinary chemical reactions or physical. It is the standard for measuring the atomic mass of the other elements. The following pages contain the bulk (but not all) of t.
Carbon Dioxide And Water Are Two Examples, But Answers Will Vary.
A) heterogeneous mixture b) solution (solid, of copper & zinc) c) solution. Web our resource for modern chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. Rutherford’s experiment involved shooting a beam of particles at a thin sheet of metal foil. Step 2 of 3 (or) step 3 of 3
The Presentation Of Information In This Chapter Assumes That You Can Already Perform.
Boils at 100°c and freezes at 0°c 3. The value of amu is given by as follows: Law of conservation of mass, law of definite proportions, and law. Web 4 chapter 3 stoichiometry.