Chemistry Chapter 1 Review
Chemistry Chapter 1 Review - 62 ly 14 13 ghq 10 cstlioo hmo sq Advanced theories of covalent bonding 6:. Element, compound, homogeneous mixture (solution), or heterogeneous mixture: Web 1.1 chemistry in context. A measure of the force of gravity between two objects. Paul wenthold topic hierarchy chapter 1: (b) theory (a widely accepted explanation of. For every reaction there is an equal and opposite reaction. Even if you’re studying for the general chemistry. Click the card to flip 👆.
Distinguish between the physical and chemical properties of matter. Paul wenthold topic hierarchy chapter 1: Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: Study of the composition of matter and the changes it undergoes. The branch of chemistry that includes the study of materials and processes that occur in living things is. 62 ly 14 13 ghq 10 cstlioo hmo sq Atoms, molecules, and ions 3: Click the card to flip 👆. Web 1.1 chemistry in context 1.2 phases and classification of matter 1.3 physical and chemical properties 1.4 measurements 1.5 measurement uncertainty, accuracy, and precision 1.6 mathematical treatment of. Web this video tutorial study guide review is for students who are taking their first semester of college general chemistry, ib, or ap chemistry.
Click the card to flip 👆. Electronic structure and periodic properties 4: Chemical bonding and molecular geometry 5: More about atoms moles and molar mass isotopes unit 3: Organic chemistry for engineers (1st semester) fall 2014: Click the card to flip 👆. Web chemistry chapter 1 review. A measure of the force of gravity between two objects. Web review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium. Place a glass of water outside.
Chemistry Chapter 1 Lesson Video YouTube
Paul wenthold topic hierarchy chapter 1: Web definition 1 / 92 mass/space click the card to flip 👆 flashcards learn test match created by sig_campbell terms in this set (92) matter is anything that has ______and occupies _________ mass/space chemistry is the study of the_____ of matter and the _____that matter undergoes composition/ changes chemistry. Electronic structure and periodic properties.
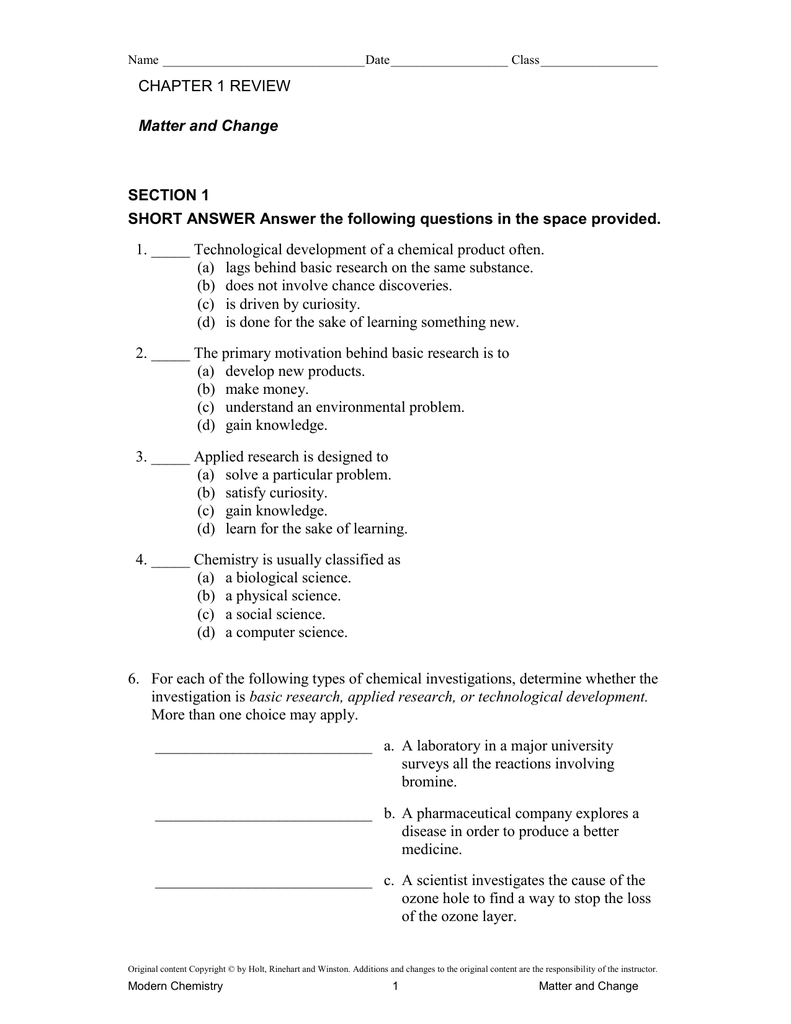
Matter and Change, pp. 14
Electronic structure and periodic properties 4: Web chapter 1 review questions number the items below in the correct order of organization, from simplest (1) to most complex (7) __ organ system __ atoms __ tissues __ cells __ organism __ organs __. Distinguish between the physical and chemical properties of matter. Chemical reactions involve processes in which reactants. 62 ly.
Chapter 1 Review
Deals with composition, structure, and reactions of matter. Atoms, molecules, and ions 3: (b) theory (a widely accepted explanation of. The branch of chemistry that includes the study of materials and processes that occur in living things is. Element, compound, homogeneous mixture (solution), or heterogeneous mixture:
Chem Section Reviews Atomic Mass Unit Ion
Click the card to flip 👆. Also explore over 389 similar quizzes in. Explain how you could experimentally determine whether the outside temperature is higher or lower than 0 °c (32 °f) without using a thermometer. Distinguish between the physical and chemical properties of matter. Course chemistry 1 (chem 1010).
modern chemistry chapter 1 test
Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: Click the card to flip 👆. Web chemistry chapter 1 review answers. Distinguish between the physical and chemical properties of matter. Deals with composition, structure, and reactions of matter.
Class 11 Chemistry Chapter 1 Exercise Exercise Poster
(a) law (states a consistently observed phenomenon, can be used for prediction); It will freeze if the temperature is below 0 °c. Web general chemistry i (chem 1210) students shared 159 documents in this course. Advanced theories of covalent bonding 6:. The weight of an object depends on the distance of the object from the center.
chapter 1 chemistry
The branch of chemistry that includes the study of materials and processes that occur in living things is. Paul wenthold topic hierarchy chapter 1: (a) law (states a consistently observed phenomenon, can be used for prediction); University university of massachusetts global; Even if you’re studying for the general chemistry.
Chemistry Chapter 1 Review Crossword Labs
Web a kilogram is defined as the mass of 1 l of water at 4°c (water freezes at 0° c). (a) law (states a consistently observed phenomenon, can be used for prediction); Web a central concept of the kinetic theory, one of the big ideas of chemistry, is the belief that. It will freeze if the temperature is below 0.
Chemistry Chapter 1 Question Answers
Web 1.1 chemistry in context 1.2 phases and classification of matter 1.3 physical and chemical properties 1.4 measurements 1.5 measurement uncertainty, accuracy, and precision 1.6 mathematical treatment of. More about atoms moles and molar mass isotopes unit 3: Web this video tutorial study guide review is for students who are taking their first semester of college general chemistry, ib, or.
Chemistry 11 chapter 1 notes
Organic chemistry for engineers (1st semester) fall 2014: A measure of the force of gravity between two objects. Web a kilogram is defined as the mass of 1 l of water at 4°c (water freezes at 0° c). The weight of an object depends on the distance of the object from the center. Element, compound, homogeneous mixture (solution), or heterogeneous.
Click The Card To Flip 👆.
The weight of an object depends on the distance of the object from the center. Web review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium. Web chemistry chapter 1 review. It will freeze if the temperature is below 0 °c.
Distinguish Among Homogeneous And Heterogeneous Mixtures, Elements, And Compounds.
University university of massachusetts global; Electronic structure and periodic properties 4: A measure of the force of gravity between two objects. Paul wenthold topic hierarchy chapter 1:
(B) Theory (A Widely Accepted Explanation Of.
Deals with composition, structure, and reactions of matter. Explain how you could experimentally determine whether the outside temperature is higher or lower than 0 °c (32 °f) without using a thermometer. Web general chemistry i (chem 1210) students shared 159 documents in this course. Web this video tutorial study guide review is for students who are taking their first semester of college general chemistry, ib, or ap chemistry.
Chemical Reactions Involve Processes In Which Reactants.
Course chemistry 1 (chem 1010). Distinguish between the physical and chemical properties of matter. Study of the composition of matter and the changes it undergoes. The branch of chemistry that includes the study of materials and processes that occur in living things is.