Chapter 8 Study Guide Covalent Bonding
Chapter 8 Study Guide Covalent Bonding - Web study with quizlet and memorize flashcards containing terms like covalent bond, dipole, double bond and more. A tightly bound group of atoms that behaves as a unit and has a positive or negative charge and behaves a unit. The energy needed to break covalent bond. Web a covalent bond in which one atom provides both bonding electrons. • they can’t give away electrons to bond. Learn vocabulary, terms, and more with flashcards, games, and other study. Molecule consisting of two of the same atoms. Web a bond formed by sharing three pairs of electrons. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes covalent bonds study guide has everything you need to ace quizzes, tests, and essays. A covalent bond in which one atom contributesboth bonding electrons.
Web chapter 8 study guide: Web chapter 8 covalent bonding study guide: Web 8 covalent bonding basics. Web atoms are held together by sharing electrons. Web about this chapter this chemistry chapter on covalent bonds will go over different forces and bonding. When covalent bonds are formed , bond dissociation energy is released and the process is. Click the card to flip 👆 definition 1 / 69 covalent bond click. Web when one atom contributes both bonding electrons in a single covalent bond, the bond is called __________. Covalent bonding term 1 / 69 when sharing of electrons occurs, the attachment between atoms that results is called a (n) ___. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes covalent bonds study guide has everything you need to ace quizzes, tests, and essays.
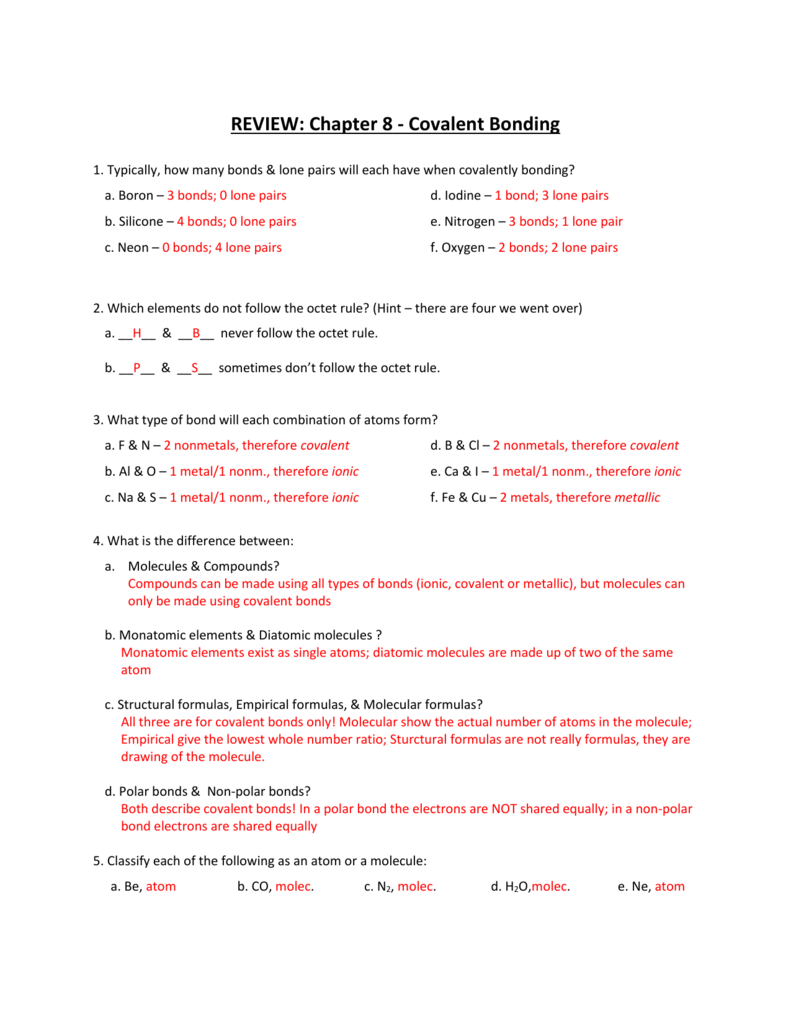
What are all the diatomic. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes covalent bonds study guide has everything you need to ace quizzes, tests, and essays. A covalent bond is characterized by the sharing of valence electrons by two adjacent atoms. Covalent bonding in this chapter: In this case the atoms usually acquire a total of eight electrons, or an octet, by sharing electrons, so that the octet rule applies. This happens most often between nonmetal elements such as carbon, hydrogen, oxygen and nitrogen. Web name _____ date _____ chapter 8 study guide covalent bonding • what is a covalent bond? Web start studying chapter 8 covalent bonding test (study guide). A tightly bound group of atoms that has either a positive or negative charge and behaves as a unit. Web covalent bonds •nonmetals hold on to their valence electrons.
30 Covalent Bonding Worksheet Answers Education Template
What are all the diatomic. Web a covalent bond in which one atom provides both bonding electrons. Web study with quizlet and memorize flashcards containing terms like covalent bond, dipole, double bond and more. Web chapter 8 identify the ions, along with their charges, in the following ionic compounds. Web from a general summary to chapter summaries to explanations of.
30 Covalent Bonding Worksheet Answers Education Template
Web chapter 8 covalent bonding study guide: Neutral group of atoms joined together by covalent bonds. Web covalent bonds •nonmetals hold on to their valence electrons. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes covalent bonds study guide has everything you need to ace quizzes, tests, and essays. In this case the atoms.
Chapter 8 Study Guide Covalent Bonding Answer Key Study Poster
Neutral group of atoms joined together by covalent bonds. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes covalent bonds study guide has everything you need to ace quizzes, tests, and essays. Web study with quizlet and memorize flashcards containing terms like covalent bond, dipole, double bond and more. • get it by sharing.
30 Covalent Bonding Worksheet Answers Education Template
A tightly bound group of atoms that has either a positive or negative charge and behaves as a unit. Covalent bonding in this chapter: • what are the characteristics of molecular compounds (covalently bonded compounds) characteristics of ionic and covalent compounds characteristics ionic compound covalent compound representative element formula unit bond. Web when one atom contributes both bonding electrons in.
Chemistry Chapter 8 Covalent Bonding Worksheet Answers / Ionic And
A tightly bound group of atoms that behaves as a unit and has a positive or negative charge and behaves a unit. Web when one atom contributes both bonding electrons in a single covalent bond, the bond is called __________. When covalent bonds are formed , bond dissociation energy is released and the process is. • what are the characteristics.
Chemistry Chp 8 Covalent Bonding Study Guide
Learn vocabulary, terms, and more with flashcards, games, and other study. Web combinations of atoms of the nonmetals and metalloids in groups 4a, 5a, 6a, and 7a of the periodic table are likely to form covalent bonds. What are all the diatomic. Neutral group of atoms joined together by covalent bonds. • what are the characteristics of molecular compounds (covalently.
Chapter 8 Study Guide Covalent Bonding Answer Key Study Poster
Bond formed between two or more atoms by a sharing of electrons metallic bond : Web 8 covalent bonding basics. Covalent bonding term 1 / 69 when sharing of electrons occurs, the attachment between atoms that results is called a (n) ___. When covalent bonds are formed , bond dissociation energy is released and the process is. Web chapter 8.
Chemistry Chp 8 Covalent Bonding Study Guide
The energy needed to break covalent bond. Web atoms are held together by sharing electrons. Web 8 covalent bonding basics. Click the card to flip 👆 definition 1 / 69 covalent bond click. When covalent bonds are formed , bond dissociation energy is released and the process is.
PPT Chapter 8 Covalent Bonding PowerPoint Presentation, free
For example, consider a simple covalently bonded. Molecule consisting of two of the same atoms. • what are the characteristics of molecular compounds (covalently bonded compounds) characteristics of ionic and covalent compounds characteristics ionic compound covalent compound representative element formula unit bond. Web a bond formed by the sharing of electrons between atoms. A tightly bound group of atoms that.
PPT Covalent Bonding Chapter 8 PowerPoint Presentation, free download
For example, consider a simple covalently bonded. Web combinations of atoms of the nonmetals and metalloids in groups 4a, 5a, 6a, and 7a of the periodic table are likely to form covalent bonds. A molecule consisiting of two atoms. Web when one atom contributes both bonding electrons in a single covalent bond, the bond is called __________. A covalent bond.
• What Are The Characteristics Of Molecular Compounds (Covalently Bonded Compounds) Characteristics Of Ionic And Covalent Compounds Characteristics Ionic Compound Covalent Compound Representative Element Formula Unit Bond.
Neutral group of atoms joined together by covalent bonds. For example, consider a simple covalently bonded. Web a bond formed by the sharing of electrons between atoms. Bond formed between two or more atoms by a sharing of electrons metallic bond :
Web Combinations Of Atoms Of The Nonmetals And Metalloids In Groups 4A, 5A, 6A, And 7A Of The Periodic Table Are Likely To Form Covalent Bonds.
A tightly bound group of atoms that behaves as a unit and has a positive or negative charge and behaves a unit. A tightly bound group of atoms that has either a positive or negative charge and behaves as a unit. Web about this chapter this chemistry chapter on covalent bonds will go over different forces and bonding. A covalent bond is characterized by the sharing of valence electrons by two adjacent atoms.
Greater Amount Of Energy Is Required To Break A Bond In Reactants Than Is Released When The New Bonds.
This happens most often between nonmetal elements such as carbon, hydrogen, oxygen and nitrogen. Bonding in which the bonding electrons are relatively free to move throughout the 3d structure electron dot. Web when one atom contributes both bonding electrons in a single covalent bond, the bond is called __________. Web atoms are held together by sharing electrons.
• They Can’t Give Away Electrons To Bond.
Web study with quizlet and memorize flashcards containing terms like what are the differences between ionic and covalent bonds?, which bonds are stronger?, which bonds have higher melting points? When h+ forms a bond with h2o the form the hydronium ion h3o+, this bond is called a coordinate covalent bond because ______. Web start studying chapter 8 covalent bonding test (study guide). What are all the diatomic.