Can Metals Form Covalent Bonds
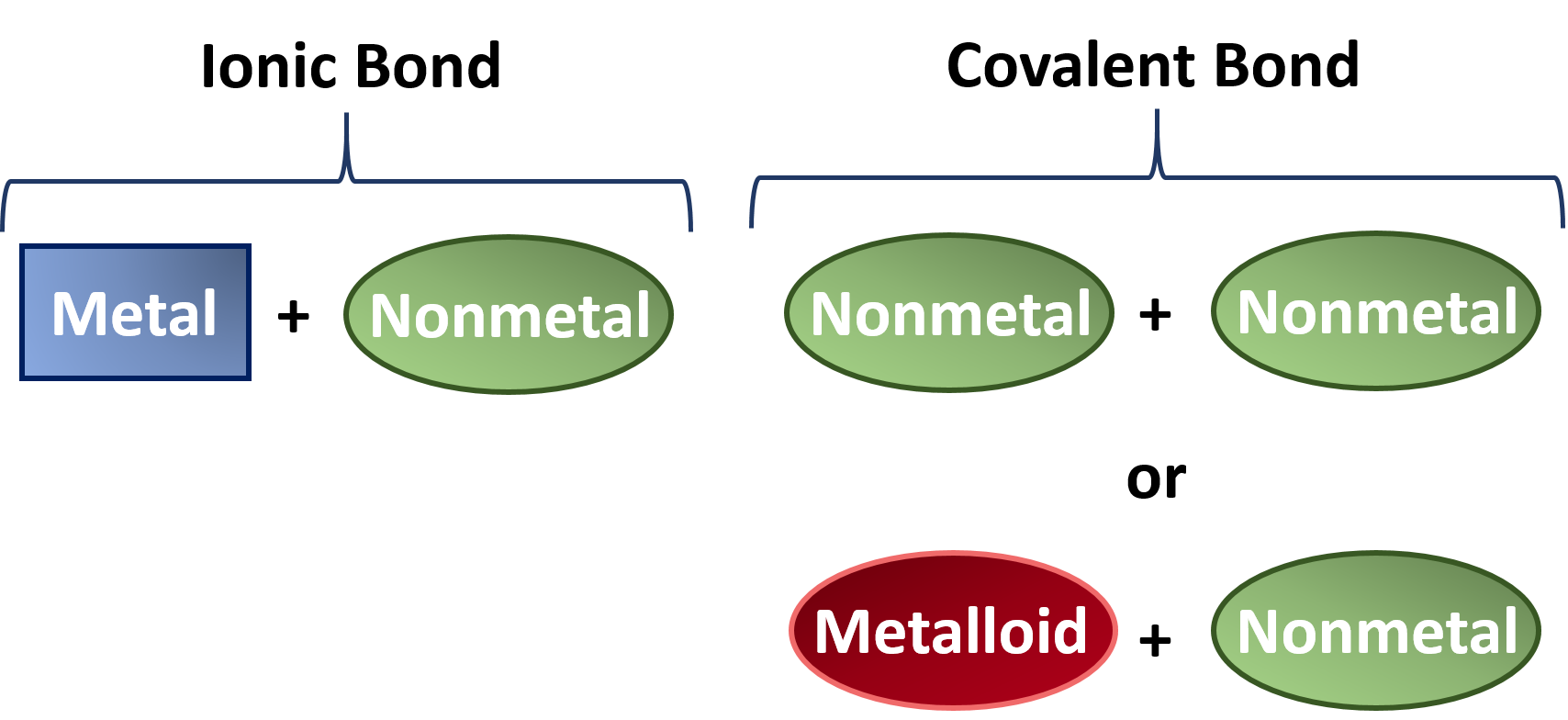
Can Metals Form Covalent Bonds - Web what i mean is can a metal react with another metal to form a compound? A metallic bond behaves more like one big molecule (except that unlike. Web metals simply do not hold on to electrons with enough strength to form much in the way of covalent bonds. Web in ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged. Web the hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. Web by contrast, for the hp12 structure, two neighboring w atoms are isolated without charge hybridization to form the covalent bonds, and, accordingly, their phonon modes. Web both metals and nonmetals can form covalent bonds, but nonmetals do so more often. Web covalent bonds consist of pairs of electrons shared by two atoms, and bind the atoms in a fixed orientation. These electron pairs are known as shared pairs or bonding pairs. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded.
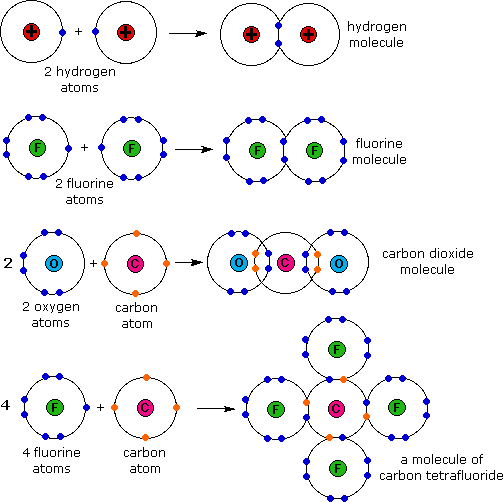
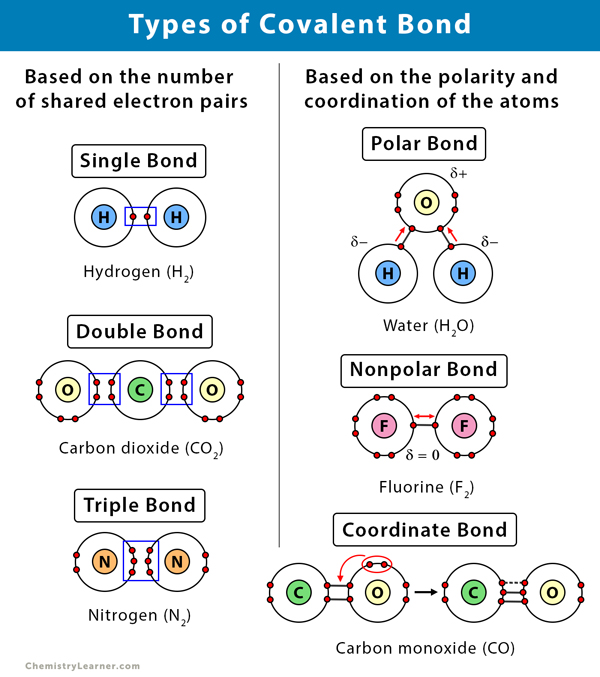
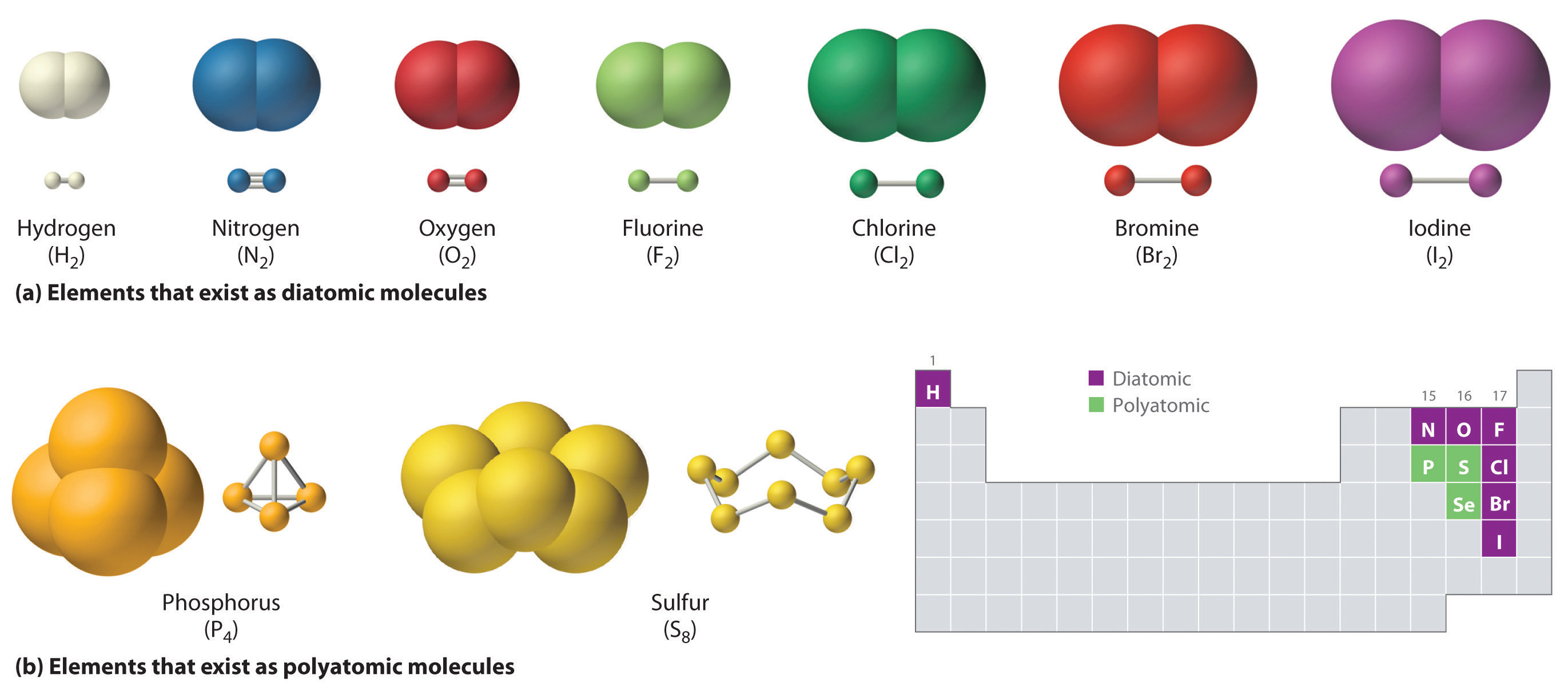
Web what i mean is can a metal react with another metal to form a compound? Web when two oxygen atoms bond, they become a molecule and don’t interact much with other molecules. Metallic bonds exist in metal crystal lattices. For a covalent bond to form, we need two atoms that both attract. It takes two electrons to. These electron pairs are known as shared pairs or bonding pairs. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. For instance, copper can form [cu(hx2o)x6]x2+ [ c u ( h x 2 o).
Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Metallic bonds exist in metal crystal lattices. Web covalent bonding generally happens between nonmetals. However, the carbon, oxygen, and nitrogen atoms can bond. [duplicate] closed 8 years ago. It takes two electrons to. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. But in other compounds containing a rwo or a few metal atoms, they can be covalently bonded. Web how can transition metals form so many bonds with ligands? Web what i mean is can a metal react with another metal to form a compound?
ASSTUDYPEACH Covalent Bonds Sharing Is Caring!
Web what i mean is can a metal react with another metal to form a compound? Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Web covalent bonds consist of pairs of electrons shared by two atoms, and bind the atoms in a fixed orientation. Web nonmetal atoms frequently form covalent bonds with.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web what i mean is can a metal react with another metal to form a compound? Web the hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. An atom that shares one or more of its. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen.
Naming covalent compounds worksheet Scientific Worksheets
These electron pairs are known as shared pairs or bonding pairs. Web covalent bonding generally happens between nonmetals. Web both metals and nonmetals can form covalent bonds, but nonmetals do so more often. Web how can transition metals form so many bonds with ligands? [duplicate] closed 8 years ago.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web in ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged. Web by contrast, for the hp12 structure, two neighboring w atoms are isolated without charge hybridization to form the covalent bonds, and, accordingly, their phonon modes. Web when two oxygen atoms bond, they become a.
How Many Single Bonds Can Carbon Form fredhughesdesign
Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Web covalent bonding generally happens between nonmetals. [duplicate] closed 8 years ago. Web in ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged. Web how can transition metals form so.
Covalent Bonding (Biology) — Definition & Role Expii
Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Web transition metals are defined as those elements that have (or readily form) partially filled d orbitals. The electrons involved are in the outer shells of the atoms. But in other compounds containing a rwo or a few metal atoms, they can be covalently.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web how can transition metals form so many bonds with ligands? Web by contrast, for the hp12 structure, two neighboring w atoms are isolated without charge hybridization to form the covalent bonds, and, accordingly, their phonon modes. Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. For a covalent bond to form, we.
Covalent Bond
Web covalent bonds consist of pairs of electrons shared by two atoms, and bind the atoms in a fixed orientation. Web transition metals are defined as those elements that have (or readily form) partially filled d orbitals. Web metals simply do not hold on to electrons with enough strength to form much in the way of covalent bonds. Web when.
chemistry picture
For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Web covalent bonding generally happens between nonmetals. Metallic bonds exist in metal crystal lattices. Web the hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. The electrons involved are in the outer shells of the.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Web when two oxygen atoms bond, they become a molecule and don’t interact much with other molecules. [duplicate] closed 8 years ago. Web metals simply do not hold on to electrons with enough strength to form much in the way of covalent bonds. A metallic bond behaves more like one big molecule (except that unlike. Web the hydrogen atom and.
A Metallic Bond Behaves More Like One Big Molecule (Except That Unlike.
For a covalent bond to form, we need two atoms that both attract. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. [duplicate] closed 8 years ago. Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion.
For Instance, Copper Can Form [Cu(Hx2O)X6]X2+ [ C U ( H X 2 O).
Web the hydrogen atom and the halogen atoms form only one covalent bond to other atoms in stable neutral compounds. Web how can transition metals form so many bonds with ligands? Web both metals and nonmetals can form covalent bonds, but nonmetals do so more often. Web covalent bonding generally happens between nonmetals.
But In Other Compounds Containing A Rwo Or A Few Metal Atoms, They Can Be Covalently Bonded.
An atom that shares one or more of its. Web what i mean is can a metal react with another metal to form a compound? Metallic bonds exist in metal crystal lattices. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms.
Web When Two Oxygen Atoms Bond, They Become A Molecule And Don’t Interact Much With Other Molecules.
Web metals simply do not hold on to electrons with enough strength to form much in the way of covalent bonds. Web transition metals are defined as those elements that have (or readily form) partially filled d orbitals. It takes two electrons to. These electron pairs are known as shared pairs or bonding pairs.