Can H2S Form Hydrogen Bonds
Can H2S Form Hydrogen Bonds - This is because for hydrogen bonding to occur there needs to be a large electronegativity difference between. Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral structure, but in solid h2s, each molecule is surrounded. The number of bonds in the compound and its type; I suggested that the difference in electronegativity between h h and s s is. H2s does not form hydrogen bonds. Web to understand the hybridization of h2s, it is vital to know two things first: Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f.
The number of bonds in the compound and its type; Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why. Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f. This is because for hydrogen bonding to occur there needs to be a large electronegativity difference between. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web to understand the hybridization of h2s, it is vital to know two things first: It is essential to know the type of. Web can h2s form a hydrogen bond? Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. I suggested that the difference in electronegativity between h h and s s is.
Web to understand the hybridization of h2s, it is vital to know two things first: Water is an excellent example of hydrogen bonding. Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. The number of bonds in the compound and its type; Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral structure, but in solid h2s, each molecule is surrounded. Web can h2s form a hydrogen bond? This is because for hydrogen bonding to occur there needs to be a large electronegativity difference between. Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f.
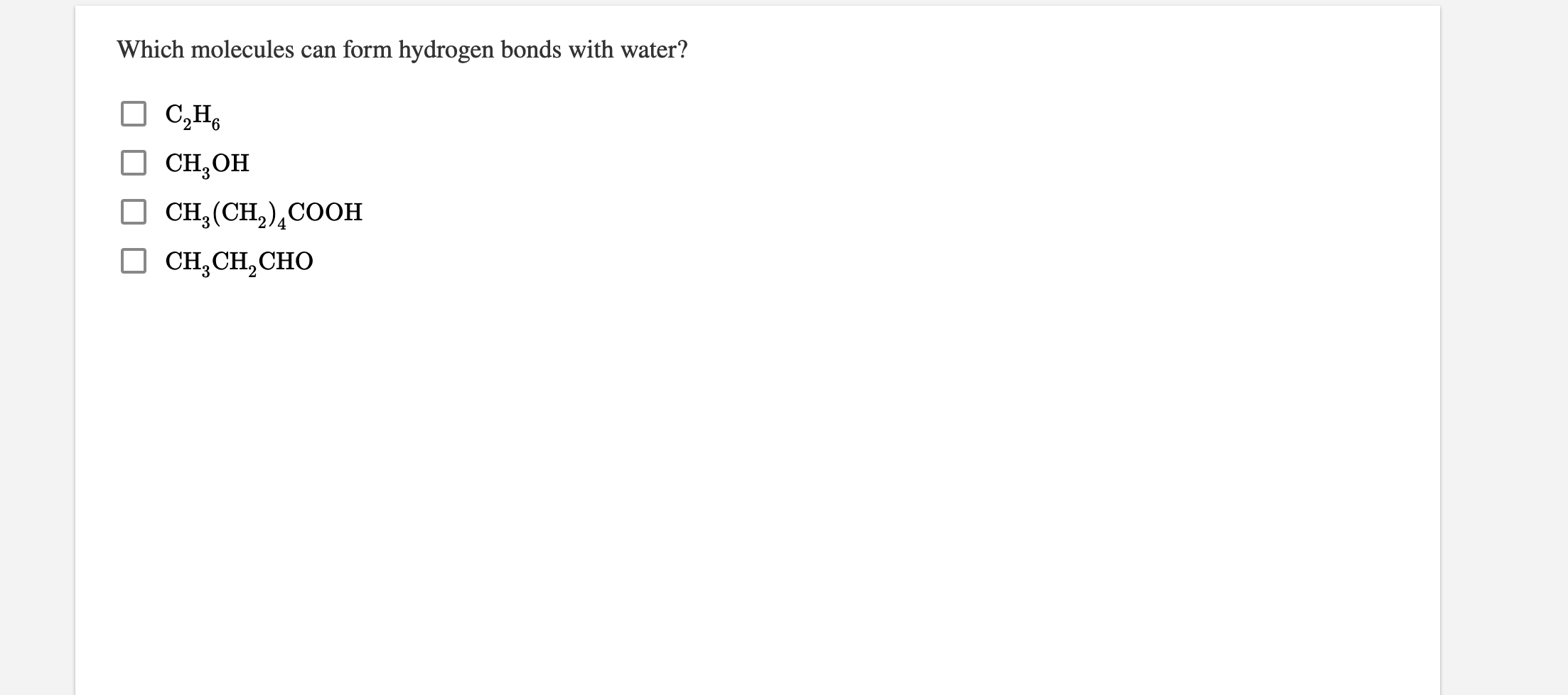
Solved Which molecules can form hydrogen bonds with water?
The bond is between the hydrogen of one water molecule and. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. Web to understand the hybridization of h2s, it is vital to know two things first: Web yes , sorry i meant h 2 s , to my mind.
Properties of Water Presentation Biology
Web examples of hydrogen bonds. Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why. Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f. Web notice that each water molecule.
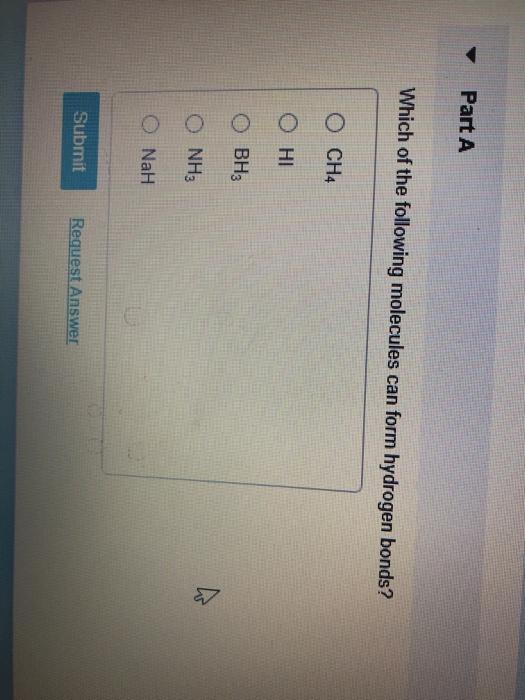
Solved Part A Which of the following molecules can form
H2s does not form hydrogen bonds. Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why. I suggested that the difference in electronegativity between h h and s s is. Web examples of hydrogen bonds. It is essential to know the type of.
Hydrogen Bonding Powerpoint
It is essential to know the type of. Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. Web examples of hydrogen.
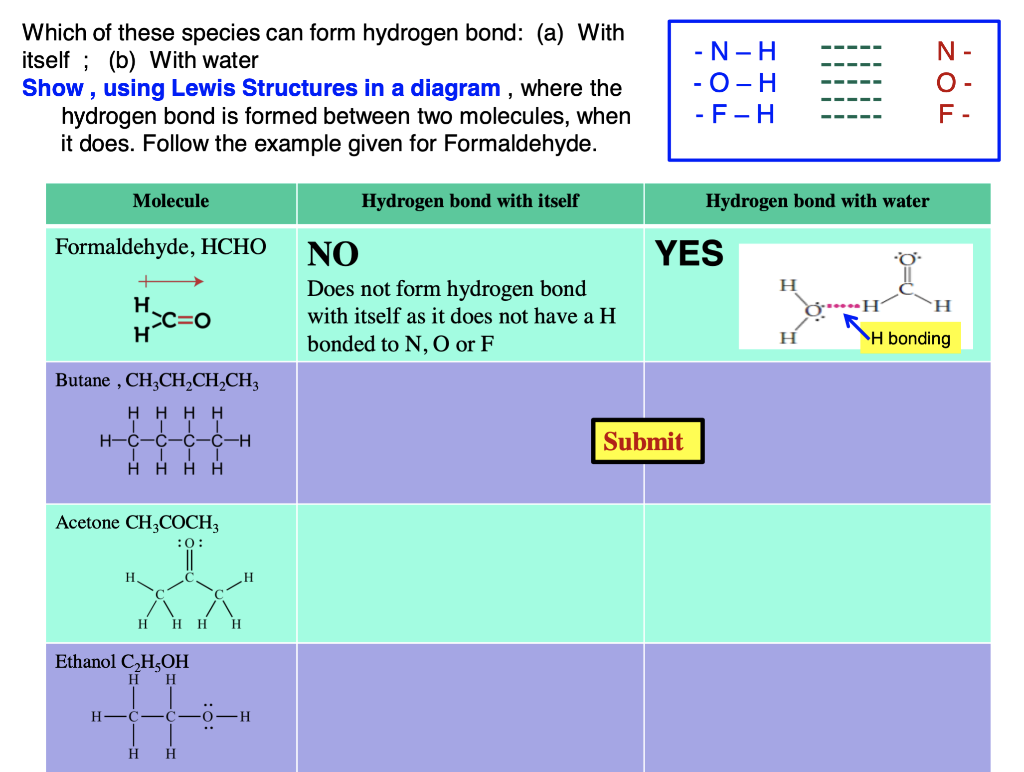
Answered Which of these species can form… bartleby
Water is an excellent example of hydrogen bonding. Web examples of hydrogen bonds. I suggested that the difference in electronegativity between h h and s s is. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. This is because for hydrogen bonding to occur there needs to be.
Hydrogen Bonds — Overview & Examples Expii
Web can h2s form a hydrogen bond? There are exactly the right numbers of δ+ hydrogens and. Water is an excellent example of hydrogen bonding. Dec 3, 2016 #4 borek mentor 29,274 3,989 what is. Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o.
Why do H2O Molecules form more Hydrogen Bonds compared to NH3 and HF
Web to understand the hybridization of h2s, it is vital to know two things first: Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f. Water is an excellent example of hydrogen bonding. The bond is between the hydrogen of one water molecule.
Hydrogen bonds YouTube
Web examples of hydrogen bonds. Web can h2s form a hydrogen bond? There are exactly the right numbers of δ+ hydrogens and. Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. The bond is between the hydrogen of one water molecule.
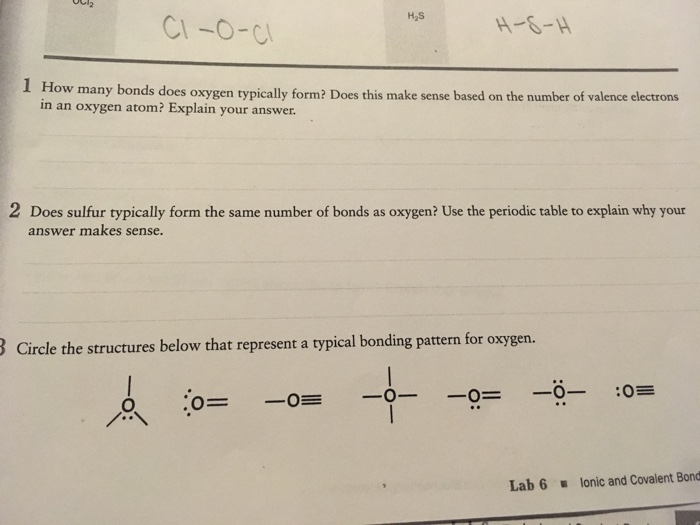
Solved H2S CIOC HSH 1 How many bonds does oxygen
I suggested that the difference in electronegativity between h h and s s is. Web examples of hydrogen bonds. There are exactly the right numbers of δ+ hydrogens and. Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. Web can h2s.
What is a hydrogen bond? [With free chemistry study guide]
Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral structure, but in solid h2s, each molecule is surrounded. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. This is because for hydrogen bonding to occur there needs to.
Web Water’s Four Hydrogen Bonds (Via Two H Atoms And Two Lone Electron Pairs) Are Evident In Ice’s Tetrahedral Structure, But In Solid H2S, Each Molecule Is Surrounded.
It is essential to know the type of. Water is an excellent example of hydrogen bonding. Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f. Web can h2s form a hydrogen bond?
Web According To The Question Hx2S H X 2 S Is Very Weakly Soluble In Water, And I Needed To Explain Why.
Due toits relatively weak intermolecular forces,. I suggested that the difference in electronegativity between h h and s s is. The bond is between the hydrogen of one water molecule and. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds.
H2S Does Not Form Hydrogen Bonds.
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. The number of bonds in the compound and its type; Dec 3, 2016 #4 borek mentor 29,274 3,989 what is. Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride.
This Is Because For Hydrogen Bonding To Occur There Needs To Be A Large Electronegativity Difference Between.
There are exactly the right numbers of δ+ hydrogens and. Web to understand the hybridization of h2s, it is vital to know two things first: Web examples of hydrogen bonds.
.PNG)




![What is a hydrogen bond? [With free chemistry study guide]](http://www.aceorganicchem.com/blog/wp-content/uploads/2018/04/4-22-2018-6-20-10-PM.jpg)