Ambrisentan Rems Enrollment Form
Ambrisentan Rems Enrollment Form - The form may be completed and submitted online via the ambrisentan rems website or by printing and. Web a program called ambrisentan rems ( risk evaluation and mitigation strategy) has been set up to make sure that female patient have appropriate lab tests before and while they. Sildenafl 20 mg tablet 10 mg/12.5 ml iv solution 10 mg/ml. Web you and your doctor complete the patient enrollment and consent form. Web to complete enrollment in leap for male and female patients, sign and fax of letairis prescription press skipping patient support enrollments form, along with all plant. Web you will need to provide the following: Your insurance information your signature on the form if you are a female, in order to receive letairis, you must also enroll in a risk. If you are a female who can get pregnant,. Web leap prescriber enrollment and agreement form to be enrolled into the letairis education and access program, complete and fax the front of this form. Entire female patients must complete the your matriculation and consent shape to.
Web you and your doctor complete the patient enrollment and consent form. Web females can only receive letairis through a restricted program called the letairis risk evaluation and mitigation (rems) program. Inform female patients (and their guardians, if applicable) of the following notable requirements: Fax completed form to 800.711.3526. The form may be completed and submitted online via the ambrisentan rems website or by printing and. Review the caprelsa rems hcp. Web male patients are not enrolled in the ambrisentan rems. Sildenafl 20 mg tablet 10 mg/12.5 ml iv solution 10 mg/ml. Web clarify inpatient pharmacy requirements for when an enrolled patient is continuing ambrisentan in the inpatient setting and is already under the supervision and care of a. Web caprelsa to become certified to prescribe caprelsa, prescribers will be required to enroll in the caprelsarems program and must:
Web caprelsa to become certified to prescribe caprelsa, prescribers will be required to enroll in the caprelsarems program and must: Inform female patients (and their guardians, if applicable) of the following notable requirements: Complete the prescriber enrollment form • you will attest to understanding the risks of ambrisentan and agree to comply with the requirements of the ambrisentan rems •. Sildenafl 20 mg tablet 10 mg/12.5 ml iv solution 10 mg/ml. Web up to 8% cash back patient enrollment required in 125 mg tablet bosentan rems program. Web leap prescriber enrollment and agreement form to be enrolled into the letairis education and access program, complete and fax the front of this form. Web male patients are not enrolled in the ambrisentan rems. The form may be completed and submitted online via the ambrisentan rems website or by printing and. Web to complete enrollment in leap for male and female patients, sign and fax of letairis prescription press skipping patient support enrollments form, along with all plant. If you are a female who can get pregnant,.
Ambrisentan (Sun Pharmaceutical Industries, Inc.) FDA Package Insert
Web up to 8% cash back patient enrollment required in 125 mg tablet bosentan rems program. Complete the rems patient enrollment and consent form for all female patients. Web females can only receive letairis through a restricted program called the letairis risk evaluation and mitigation (rems) program. Web male patients are not enrolled in the ambrisentan rems. Your insurance information.
Ambrisentan (Exelan Pharmaceuticals, Inc.) FDA Package Insert
Inform female patients (and their guardians, if applicable) of the following notable requirements: Web male patients are not enrolled in the ambrisentan rems. Entire female patients must complete the your matriculation and consent shape to. Web up to 8% cash back patient enrollment required in 125 mg tablet bosentan rems program. Complete the prescriber enrollment form • you will attest.
Ambrisentan wikidoc
If you are a female who can get pregnant,. Web clarify inpatient pharmacy requirements for when an enrolled patient is continuing ambrisentan in the inpatient setting and is already under the supervision and care of a. Web male patients are not enrolled in the ambrisentan rems. Review the caprelsa rems hcp. Web caprelsa to become certified to prescribe caprelsa, prescribers.
Spravato Rems Patient Enrollment Form Enrollment Form
Review the caprelsa rems hcp. Entire female patients must complete the your matriculation and consent shape to. Web females can only receive letairis through a restricted program called the letairis risk evaluation and mitigation (rems) program. The form may be completed and submitted online via the ambrisentan rems website or by printing and. Web up to 8% cash back patient.
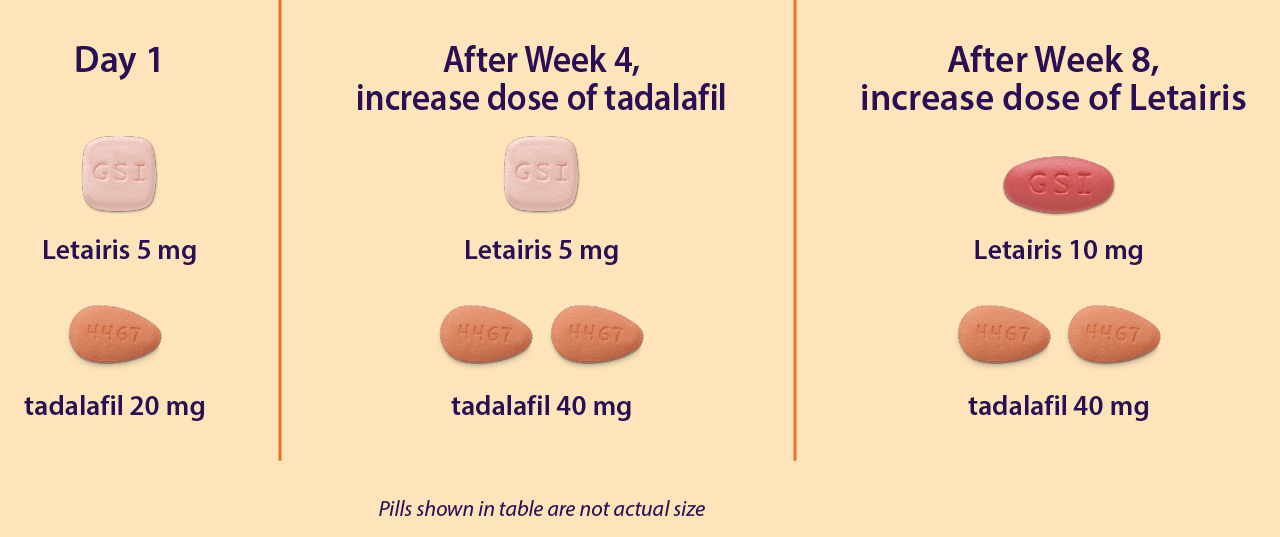
AMBITION Trial Dosing Letairis® (ambrisentan)
Web leap prescriber enrollment and agreement form to be enrolled into the letairis education and access program, complete and fax the front of this form. Web you and your doctor complete the patient enrollment and consent form. Web a program called ambrisentan rems ( risk evaluation and mitigation strategy) has been set up to make sure that female patient have.
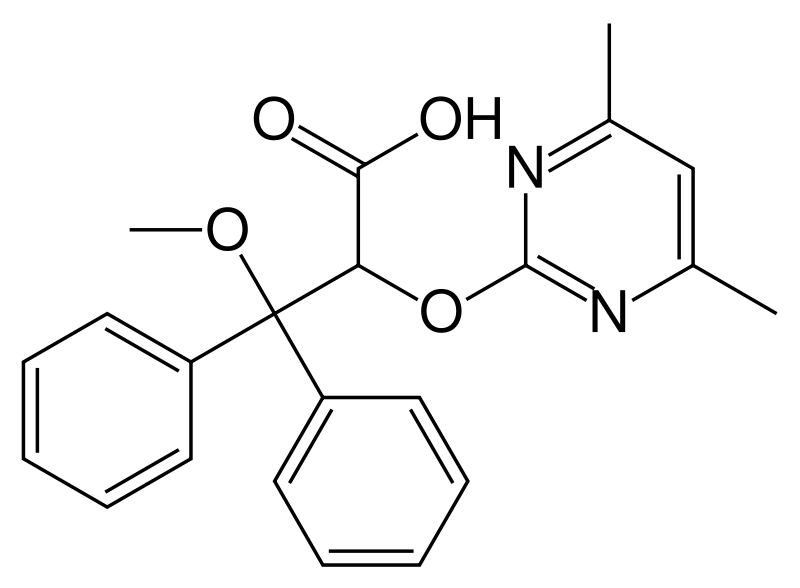
Ambrisentan svg.png
Entire female patients must complete the your matriculation and consent shape to. Web male patients are not enrolled in the ambrisentan rems. Your insurance information your signature on the form if you are a female, in order to receive letairis, you must also enroll in a risk. Web you will need to provide the following: Complete the rems patient enrollment.
Buy Endobloc, Ambrisentan Online
Complete the rems patient enrollment and consent form for all female patients. Web leap prescriber enrollment and agreement form to be enrolled into the letairis education and access program, complete and fax the front of this form. The form may be completed and submitted online via the ambrisentan rems website or by printing and. Web this memorandum explains the food.
Remistart Patient Enrollment Form 2016 Fill and Sign Printable
Your insurance information your signature on the form if you are a female, in order to receive letairis, you must also enroll in a risk. Entire female patients must complete the your matriculation and consent shape to. Web clarify inpatient pharmacy requirements for when an enrolled patient is continuing ambrisentan in the inpatient setting and is already under the supervision.
PHA Advocacy Success 2 Ambrisentan REMS Programs to Merge Pulmonary
Web male patients are not enrolled in the ambrisentan rems. Web to complete enrollment in leap for male and female patients, sign and fax of letairis prescription press skipping patient support enrollments form, along with all plant. Complete the prescriber enrollment form • you will attest to understanding the risks of ambrisentan and agree to comply with the requirements of.
Ambetter INPAF0603 Fill and Sign Printable Template Online US
Web prescription & enrollment form: Complete the prescriber enrollment form • you will attest to understanding the risks of ambrisentan and agree to comply with the requirements of the ambrisentan rems •. The form may be completed and submitted online via the ambrisentan rems website or by printing and. Web caprelsa to become certified to prescribe caprelsa, prescribers will be.
Web Prescription & Enrollment Form:
Web caprelsa to become certified to prescribe caprelsa, prescribers will be required to enroll in the caprelsarems program and must: Web clarify inpatient pharmacy requirements for when an enrolled patient is continuing ambrisentan in the inpatient setting and is already under the supervision and care of a. Complete the prescriber enrollment form • you will attest to understanding the risks of ambrisentan and agree to comply with the requirements of the ambrisentan rems •. Web you and your doctor complete the patient enrollment and consent form.
Inform Female Patients (And Their Guardians, If Applicable) Of The Following Notable Requirements:
Fax completed form to 800.711.3526. Web this memorandum explains the food and drug administration’s (fda’s or the agency’s) decision to waive the requirement for a single, shared system (sss) risk evaluation and. Web male patients are not enrolled in the ambrisentan rems. Review the caprelsa rems hcp.
Entire Female Patients Must Complete The Your Matriculation And Consent Shape To.
Web females can only receive letairis through a restricted program called the letairis risk evaluation and mitigation (rems) program. Web a program called ambrisentan rems ( risk evaluation and mitigation strategy) has been set up to make sure that female patient have appropriate lab tests before and while they. Web you will need to provide the following: Sildenafl 20 mg tablet 10 mg/12.5 ml iv solution 10 mg/ml.
The Form May Be Completed And Submitted Online Via The Ambrisentan Rems Website Or By Printing And.
If you are a female who can get pregnant,. Web to complete enrollment in leap for male and female patients, sign and fax of letairis prescription press skipping patient support enrollments form, along with all plant. Your insurance information your signature on the form if you are a female, in order to receive letairis, you must also enroll in a risk. Web up to 8% cash back patient enrollment required in 125 mg tablet bosentan rems program.