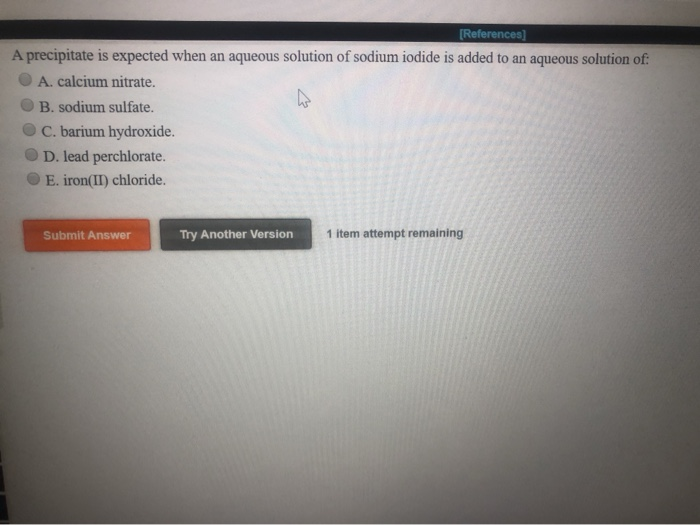
Which Anion Will Form A Precipitate With Al3+
Which Anion Will Form A Precipitate With Al3+ - A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,. Web 7 rows white precipitate remains: Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. Which pair of ions would not be expected to form a precipitate when dilute solutions of each are mixed? Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Web chemical separation by precipitation. Web potassium ferricyanide will give a brown coloration but no precipitate with fe3+ fe 3 +. Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. Click the card to flip 👆. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq).
Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. Web chemical separation by precipitation. Aluminium, al 3+ white precipitate: Whether or not such a. Web chemistry questions and answers. Web which pair of ions would not be expected to form a precipitate when solutions are mixed? Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? There are two possible reactions. A type of quantitative chemical analysis that. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3:
Click the card to flip 👆. Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. Web chemistry questions and answers. A type of quantitative chemical analysis that. Whether or not such a. Web which pair of ions would not be expected to form a precipitate when solutions are mixed? Let’s look at an example of a precipitation reaction. Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3:
Solved Which anion will form a precipitate with Ca2+? OH ОСІ
Whether or not such a. Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. Web 7 rows white precipitate remains: Web which pair of ions would not be expected to form a precipitate when solutions are mixed? A type of quantitative chemical analysis that.
Anion_Formation
Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Click the card to flip 👆. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq). Web precipitation is when a chemical reaction occurs between two solutions, and.
Solved 3. For each of the following, identify the anion or
Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq). Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. A mixture.
Solved Experiment 11 PreLab Questions Nameca rmn Section
Web which pair of ions would not be expected to form a precipitate when solutions are mixed? Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,. Web chemistry.
Solved QUALITATIVE ANALYSIS OF ANIONS EXPERIMENT 13
Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. Whether or not such a. Web chemistry questions and answers. Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic.
How Does A Precipitate Form freelancegraphicdesigndetroit
Web chemistry questions and answers. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3: Web 7 rows white precipitate remains: Let’s look at an example of a precipitation reaction. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq).
Chimie, fizică și ecologie Identificarea cationilor (reacții de
Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,. Web 7 rows white precipitate remains: Web which pair of.
Anion analysis POSTLABORATORY ASSIGNMENT 1. An iodide/chloride/sulfate
Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Web 7 rows white precipitate remains: Click the card to flip 👆. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq). A mixture of metal ions in a solution.
PPT IONS PowerPoint Presentation ID2435906
Whether or not such a. A type of quantitative chemical analysis that. Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,. Web chemistry chemistry questions and answers question.
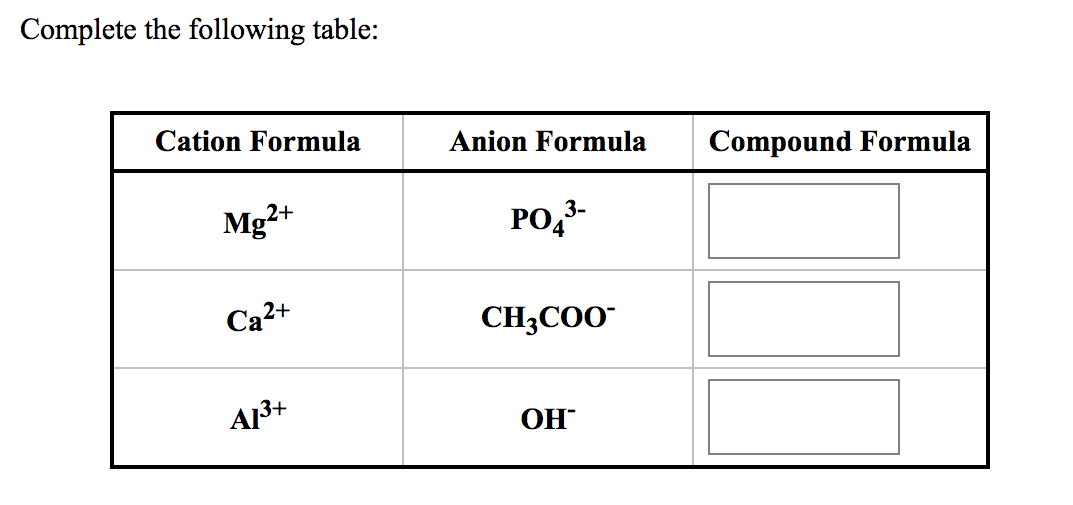
Solved Complete the following table Cation Formula Anion
Web chemistry questions and answers. Let’s look at an example of a precipitation reaction. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3: Which pair of ions would not be expected to form a precipitate when dilute solutions of each are mixed? Web which pair of ions would not be expected to form.
Web Chemistry Chemistry Questions And Answers Question 5 (10 Points) Which Anion Will Form A Precipitate With A13+?
Let’s look at an example of a precipitation reaction. Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Which pair of ions would not be expected to form a precipitate when dilute solutions of each are mixed? Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions.
Web Aluminum Ion Reacts With Aqueous Ammonia To Produce A White Gelatinous Precipitate Of Al(Oh) 3:
Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq). With fe2+ fe 2 +, a dark blue precipitate is formed. Web potassium ferricyanide will give a brown coloration but no precipitate with fe3+ fe 3 +.
A Type Of Quantitative Chemical Analysis That.
Web chemical separation by precipitation. Aluminium, al 3+ white precipitate: Web 7 rows white precipitate remains: Click the card to flip 👆.
Whether Or Not Such A.
There are two possible reactions. Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,.