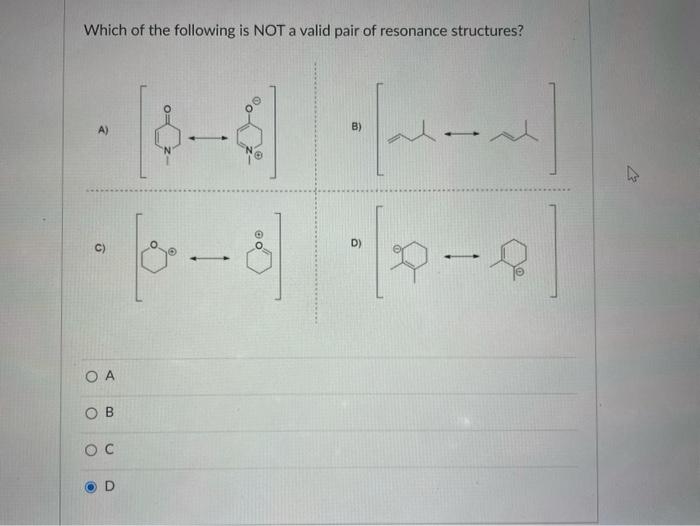
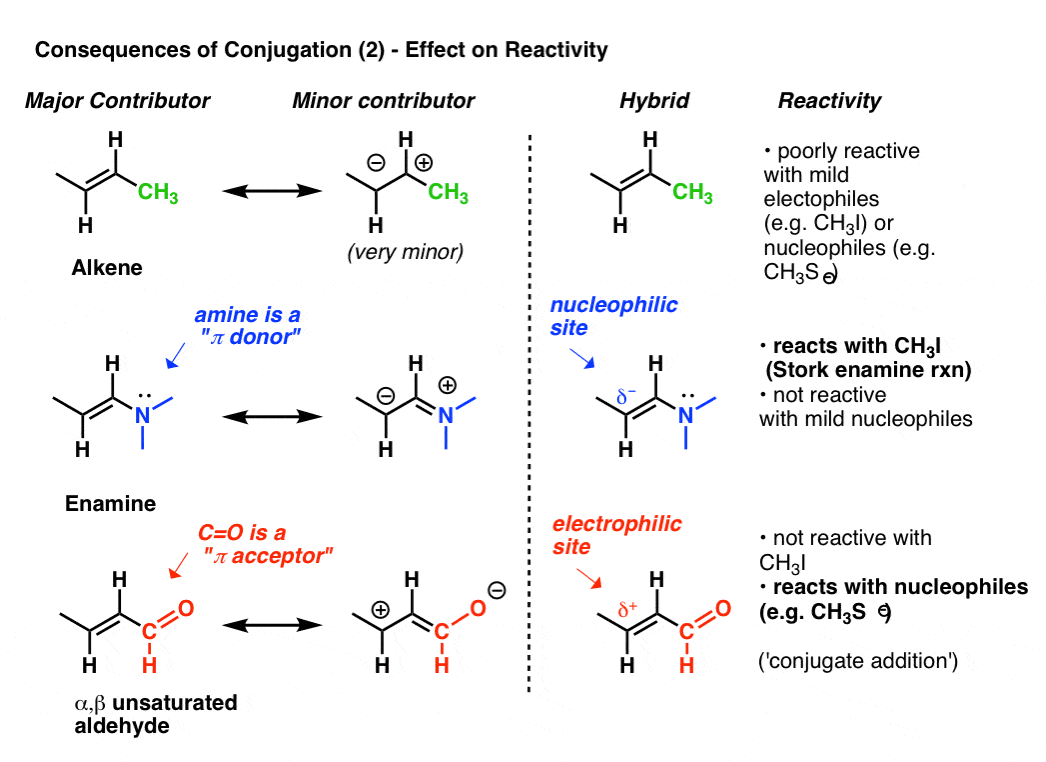
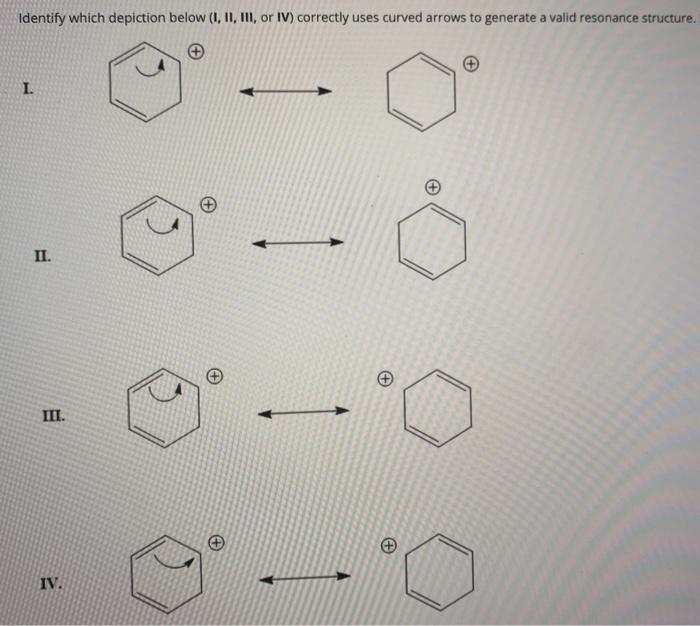
Identify The Valid Major Resonance Form Of Formaldehyde Oximate Ion.
Identify The Valid Major Resonance Form Of Formaldehyde Oximate Ion. - Web when two or more lewis structures differing only in the positions of the electrons are needed to describe a molecule, they are called resonance forms. Web last updated sep 24, 2022 2.4: Exposure to formaldehyde can cause leukemia and cancers of the nose, throat, and sinuses. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Equivalent lewis structures are called. Web structure and bonding molecular formaldehyde contains a central carbon atom with a double bond to the oxygen atom and a single bond to each hydrogen atom. Such is the case for. Explain the concept of resonance and draw lewis structures representing resonance. At concentrations above the niosh rel, or where there is no rel, at any detectable concentration: If this representation is the only correct resonance structure, we.
Web when two or more lewis structures differing only in the positions of the electrons are needed to describe a molecule, they are called resonance forms. The superposition of i and ii shows that the actual molecule has a lower. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). While both resonance structures are chemically identical, the negative charge is on a. Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. Web last updated sep 24, 2022 2.4: This report presents the results of an experimental study of a new process for the catalytic oxidation, at ambient temperatures, of dilute formaldehyde. Web we need to draw resonance structures of bicarbonate ion, formaldehyde oxime, and formaldehyde oximate ion. High levels of exposure may cause some types of cancers. Learn more from the agency for.
While both resonance structures are chemically identical, the negative charge is on a. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Such is the case for. Learn more from the agency for. Web the relative energies of the resonance forms and the actual molecule (i.e., the hybrid) are shown below. We also need to show how those structures can be. Web structure and bonding molecular formaldehyde contains a central carbon atom with a double bond to the oxygen atom and a single bond to each hydrogen atom. Equivalent lewis structures are called. Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. Web formaldehyde (gas) is on the proposition 65 list because it can cause cancer.
Chemistry Recent Questions
Web when two or more lewis structures differing only in the positions of the electrons are needed to describe a molecule, they are called resonance forms. If this representation is the only correct resonance structure, we. High levels of exposure may cause some types of cancers. Such is the case for. While both resonance structures are chemically identical, the negative.
Conjugation And Resonance In Organic Chemistry
Web when two or more lewis structures differing only in the positions of the electrons are needed to describe a molecule, they are called resonance forms. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Web minimum energy minimum energy is analogous to not drinking too much.
Four major resonance structures are possible for the following cation
Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure for a given compound. Determine the relative stability of resonance structures using a set of. While both resonance structures are chemically identical, the negative charge is on a. If you drink too much coffee in the morning, you might get too hyper.
6.2. Resonance Organic Chemistry 1 An open textbook
Web last updated jan 22, 2023 a resonance form is another way of drawing a lewis dot structure for a given compound. Drawing resonance forms objectives after completing this section, you should be able to use the concept of. If you drink too much coffee in the morning, you might get too hyper over the. Such is the case for..
Chemistry Archive April 30, 2015
We also need to show how those structures can be. Web we need to draw resonance structures of bicarbonate ion, formaldehyde oxime, and formaldehyde oximate ion. Equivalent lewis structures are called. At concentrations above the niosh rel, or where there is no rel, at any detectable concentration: Exposure to formaldehyde can cause leukemia and cancers of the nose, throat, and.
Solved 9. Which of the following is NOT a valid resonance
Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). This report presents the results of an experimental study of a new process for the catalytic oxidation, at ambient temperatures, of dilute formaldehyde. We also need.
Solved Identify which depiction below (1, 11, III, or IV)
We also need to show how those structures can be. While both resonance structures are chemically identical, the negative charge is on a. Web minimum energy minimum energy is analogous to not drinking too much coffee in the morning. Determine the relative stability of resonance structures using a set of. Web a molecule or ion with such delocalized electrons is.
Solved 9. Which of the following is NOT a valid resonance
At concentrations above the niosh rel, or where there is no rel, at any detectable concentration: Web use formal charges to identify the most reasonable lewis structure for a given molecule; Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Such is the case.
Solved Question 9 1 pts The following Lewis structure has
We also need to show how those structures can be. Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. While both resonance structures are chemically identical, the negative charge is on a. High levels of exposure may cause some types of cancers. At concentrations above the niosh rel, or where there is no rel, at any detectable.
[Solved] help please i think im wrong. Identify the valid major
Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. At concentrations above the niosh rel, or where there is no rel, at any detectable concentration: Exposure to formaldehyde can cause leukemia and cancers of the nose, throat, and sinuses. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding.
At Concentrations Above The Niosh Rel, Or Where There Is No Rel, At Any Detectable Concentration:
If you drink too much coffee in the morning, you might get too hyper over the. Web structure and bonding molecular formaldehyde contains a central carbon atom with a double bond to the oxygen atom and a single bond to each hydrogen atom. The superposition of i and ii shows that the actual molecule has a lower. Learn more from the agency for.
Web A Molecule Or Ion With Such Delocalized Electrons Is Represented By Several Contributing Structures (Also Called Resonance Structures Or Canonical Forms).
Drawing resonance forms objectives after completing this section, you should be able to use the concept of. Web formaldehyde can cause irritation of the skin, eyes, nose, and throat. Exposure to formaldehyde can cause leukemia and cancers of the nose, throat, and sinuses. Web minimum energy minimum energy is analogous to not drinking too much coffee in the morning.
Determine The Relative Stability Of Resonance Structures Using A Set Of.
This report presents the results of an experimental study of a new process for the catalytic oxidation, at ambient temperatures, of dilute formaldehyde. We also need to show how those structures can be. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Web use formal charges to identify the most reasonable lewis structure for a given molecule;
Web The Two Oxygens Are Both Partially Negative, This Is What The Resonance Structures Tell You!
Web when two or more lewis structures differing only in the positions of the electrons are needed to describe a molecule, they are called resonance forms. While both resonance structures are chemically identical, the negative charge is on a. Explain the concept of resonance and draw lewis structures representing resonance. Web the relative energies of the resonance forms and the actual molecule (i.e., the hybrid) are shown below.